| Posted: Sep 04, 2017 | |
Bioactive titanium implant material with multifunctional nano-bio-interface |
|
| (Nanowerk Spotlight) The implantation of orthopaedic devices is associated with a high risk of post-operative complications that increases substantially with each revision surgery. | |
| Revision surgeries are required primarily for two reasons: 1) implants frequently do not integrate successfully leading to loosening and; 2) bacterial infections that result in biofilm formation. | |
| In the last 10 years, there were 410,767 revision hip replacements and and 480,440 knee replacements in Australia alone (Source: The Australian Orthopaedic Association National Joint Replacement Registry, 2016 Annual Report); and the number of these replacements conducted annually continues to grow. | |
| Although the cumulative rate of revisions over a 10 year period is less than 5% for all patients, it is unacceptably high at 16% for patients suffering from bone-related conditions such as osteoporosis, osteoarthritis and ostomalacia or having poor bone structure. | |
| The situation is similar with knee replacement surgeries, with up to 10.5% of knee implants requiring revision. The significant increase in the number of patients requiring revision surgery is driven by our aging population, who more frequently have poor bone quality and worse implant integration. | |
| An international research team from Japan and Australia now have proposed a two-pronged strategy to address this outstanding clinical problem by combatting infections and providing bioactivity for titanium implants. They report their findings in Nanomaterials ("Two-in-One Biointerfaces—Antimicrobial and Bioactive Nanoporous Gallium Titanate Layers for Titanium Implants") | |
| "Our nanostructured surfaces simultaneously are highly antimicrobial as well as bioactive," Dr. Wojciech Chrzanowski, a Senior Lecturer at the Australian Institute for Nanoscale Science and Technology, tells Nanowerk. "The goal of combining both functions without inducing cytotoxicity has thus far proved elusive. Unlike other approaches that use highly toxic antimicrobial compounds and induce undesired cytotoxicity, our approach not only exhibits outstanding antimicrobial activity but also it promotes the formation of bone-like structures." | |
 |
|
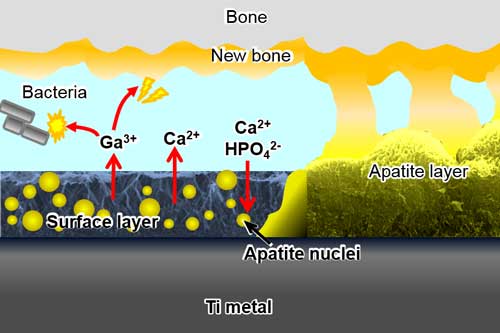
| Bioactive titanium slowly releases Ga3+ ions to inhibit adhesion and proliferation of bacteria while at the same time stimulating new bone formation. The metal also releases Ca2+ ions to form bone-like apatite on its surface and directly bond to bone in a living body. (Image: Chubu University) | |
| Yamaguchi and Chrzanowski and their collaborators have designed novel gallium-containing nanostructured interfaces that are capable of sustainably released gallium ions. | |
| "In this way, we will achieve highly desired antimicrobial activity without compromising the ability of the implant to bind to bone," Seiji Yamaguchi, a Lecturer at Chubu University, Department of Biomedical Sciences, and the paper's first author, points out. "In fact, gallium ions are likely to further improve bone bonding ability as they show no cytotoxicity and strikingly enhance mineralization of the matrix directly on the surface, thus supporting bone tissue formation." | |
| The team's innovative technology is very simple, applicable to any shape and size of the implant, and inexpensive. Furthermore, it does not require any sophisticated chemistries or instrumentation, all of which is a significant advantage from the commercialization point of view. | |
| The major novelty of the work is its two-pronged strategy: co-presentation of ions and structure that regulate bioactivity and which provides high antimicrobial activity. | |
| Target applications for this work are titanium implants such as bone plate, intramedullary nails, bone screw, and spinal implants such as spinal discs. The 3D-printing of this kind of implants has become commonplace and the geometries of implants has become relatively complex. | |
| These complexities make traditional approaches of surface modification such as PVD or CVD not applicable. The Australian team's method is fully compatible with any size and geometry and all currently used titanium alloys. | |
| "These features make the technology particular attractive for industry," notes Yamaguchi. "We are currently fast-tracking our technology towards towards large animal studies and commercialization with a Japanese-based implant producer." | |
| Going forward, the team is pursuing work in three areas. Firstly, the design of the surface modification methodology which resulted in a) desired nanostructures, and b) the incorporation of gallium which is slowly but sustainably released in simulated body conditions. Secondly, conformation of antimicrobial activity using the most challenging type of multidrug resistant bacteria. And lastly, conformation of bioactivity using ISO standard tests. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
