| Nov 28, 2018 | |
'Artificial leaf' uses visible light to accelerate chemical reactions |
|
| (Nanowerk Spotlight) Photocatalysis using sunlight has attracted considerable research attention in the drive away from fossil fuels and towards the vision of a solar-powered chemical manufacturing industry. | |
| Over the past several years, metal nanoparticles photosensitization over semiconductors with a large band gap has emerged as a promising strategy for developing visible-light responsive photocatalytic materials. | |
| Most existing photocatalysts are based on ruthenium or iridium designer organo-metallic complexes, which have very low optical cross-sections for visible light, i.e. they can absorb only a fraction of the incident light. | |
| "Although light absorption is an intrinsic step in photocatalysis, existing approaches rely on poorly absorbing catalysts that require high illumination intensities to afford enhanced efficiencies," Daniel E. Gómez, an associate professor who runs the Polaritonics Lab at RMIT University, explains to Nanowerk. | |
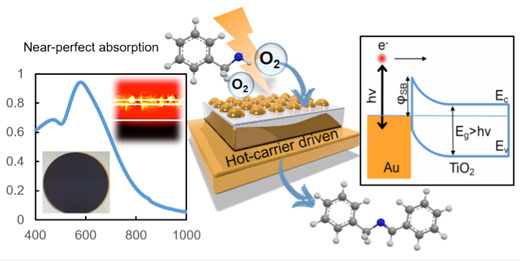
| In a study (ACS Catalysis, "Hot-Carrier Organic Synthesis via the Near-Perfect Absorption of Light"), Gómez and his team together with scientists from CSIRO, demonstrate a new plasmonic metamaterial that can absorb nearly 100% of incident light (of a specific color) and use this energy to accelerate the production of chemicals. | |
| In constructing the near-perfect absorber, the researchers employ gold nanoparticles, TiO2 as the spacer layer, and a continuous gold thin-film as the rear reflective layer. The TiO2/Au nanoparticles interface acts as a Schottky contact through which hot electrons with energies in excess of this barrier can be emitted. The resulting charge-separated states (electrons in TiO2 and holes in Au) can subsequently be used for reductive and oxidative chemical transformations, respectively. | |
| This material can be thought of as a kind of an artificial leaf, that when inserted into a container with a mix of chemicals, uses light to initiate and accelerate chemical transformations. | |
 |
|
| Schematic depiction of the hot-carrier organic synthesis via the near-perfect absorption of light including a TiO2/Au nanoparticle contact energy diagram. (Image: RMIT University) | |
| In their tests, the research team showed that the light-driven reactions can take place at ambient temperature (i.e. no extra heating required) and ambient atmospheric pressure, using a conventional $3 white-light LED as the only power source. | |
| Specifically, this mirror/semiconductor/nanoparticle metamaterial can absorb ~94% of incident photons at the wavelength of its plasmon resonance. It achieves a catalytic rate of 37 mmolproduct/gAu catalyst/h for the selective oxidative coupling of amines to imines on the metamaterial surface, representing a ∼29-fold increase in efficiency over traditional semiconductor/nanoparticles contacts. | |
| "With hot-carrier extraction from plasmonic structures attracting significant attention due to their increasing role in unique photocatalytic transformation pathways, we believe no previous study comprises a set of experimental and theoretical results as consistent and homogenous as those presented here on the subject," notes Gómez. "Our results will stimulate further work on hot-carrier photocatalysis and plasmon-hot-carrier extraction, interdisciplinary fields that are currently undergoing rapid growth." | |
| The team's proof-of-concept demonstration means that in the future, organic reactions – such as those used for making medicinal compounds – could be driven with this new catalytic system under mild visible light irradiation. | |
| Compared with traditional catalytic processes that require intensive external heating and pressure as the driving force for the reaction, this novel catalytic system could maximize light absorption and efficiently convert photon energy into chemical energy. | |
| Previously, Gómez and his team have developed several systems, which show excellent near-perfect light absorption. We have reported on an example of this work in a previous Nanowerk Spotlight: "Black gold maximizes the light absorption of nanomaterials". In principle, many complex organic reactions could take place via charge transfers and exploring these possibilities drives the scientists' work. | |
| "Our ultimate goal is to use this nanotechnology to harness sunlight efficiently and convert solar energy into chemicals under renewable and sustainable conditions," says Gómez. "We are continuing to work on more complex near-perfect absorption systems with tunable light absorption property and catalytic active sites for future applications." | |
| He cautions that currently one of the limiting factors of this novel system for applications in chemical syntheses is mass-transfer and efficient mixing of chemicals – though the team believes that the incorporation of their technology with photoredox flow-chemistry approaches is the most likely solution to this problem. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
