| Dec 06, 2018 | |
Switching peroxidase activity of gold nanoparticles to detect proteolytic biomarker |
|
| (Nanowerk Spotlight) Nanoparticles that mimic the complexity and function of natural enzymes can act as effective peroxidase to catalyse for the oxidization of 3,3’,5’5-tetramethylbenzidine (TMB), generating an oxidised blue-coloured product. | |
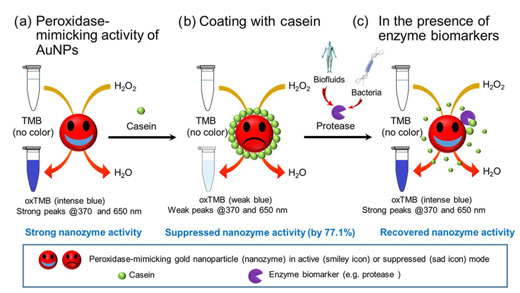
| For the first time scientists at the Institute for Global Food Security, the Queen’s University of Belfast found that when the surface of gold nanoparticle (AuNPs) is coated with casein, the intrinsic peroxidase-mimicking activity of AuNPs is suppressed strongly, i.e. by up to 77.1%, due to the surface shielding effects. | |
| However, in the presence of protease, the casein shielding layer is degraded, allowing the recovery of the peroxidase-mimicking activity of the AuNPs and thereby the detection of the proteolytic enzyme. | |
| The involvement of proteases in all life functions has highlighted their crucial role in the progression of many diseases and in microorganism growth. Conventional methods for protease detection are slow, lack the sensitivity required for low level detection, and cannot be utilised on-site. These limitations hinder the applicability of these assays to provide an early indication of food spoilage, infection and disease state. | |
| With the need to overcome these limitations, Dr. Cuong Cao’s research group at the Institute for Global Food Security at the Queen’s University of Belfast developed a simplified approach utilising the properties of gold nanoparticles. | |
| The results highlighted a sensitive, fast and cost effective nanosensor for protease detection in milk and urine. This work has recently been published as an open access article in Nano Research ("Unusual Switchable Peroxidase-mimicking Nanozyme for the Determination of Proteolytic Biomarker"). | |
 |
|
| Overall scheme demonstrating the switching of peroxidase-mimicking activity of casein coated AuNPs for the detection of enzyme biomarkers. (a) AuNPs possess the peroxidase-mimicking ability to catalyse the oxidation of TMB in the presence of H2O2, resulting in oxTMB which exhibits a blue color and strongly absorbs light at 370 and 650 nm. (b) When casein is coated onto the AuNP surface, this peroxidase-mimicking activity is suppressed, resulting in a weakened absorbance at 370 and 650 nm. (c) In the presence of protease derived from bio-fluids or microorganisms, hydrolytic cleavage of the surface shielding layer (casein) results in the recovery of the peroxidase-mimicking activity of AuNP. (© Springer) (click on image to enlarge) | |
| To create the nanosensor, the investigators first determined that under predefined conditions AuNPs were able to mimic the behaviour of natural enzymes, i.e. the peroxidase enzyme that can speed up the oxidization of 3,3’,5’5-tetramethylbenzidine (TMB) to produce a blue coloured product which strongly absorbs light at 370 nm and 650 nm and clearly visible via the naked-eye. | |
| Interestingly, the investigators also found that this behaviour could be tuned to suit the intended application. | |
| In order to develop this finding into a protease detection approach, the AuNP surface was coated with casein (a protein present substantially in milk) which supressed the enzyme-mimicking behaviour by 77.1%. | |
| This decrease was due to the casein molecule blocking surface reactions, meaning the reactions happened much slower (no vivid blue colour formation). | |
| Significantly, casein also acted as a substrate for protease digestion, resulting in its removal from the AuNP surface and subsequent vivid blue colour formation due to the recovery of enzyme-mimicking behaviour. | |
| The investigators also proposed to use the peak absorbance at 370 nm to quantify the peroxidase-mimicking activity of AuNPs. This is because in a medium with high electrolyte content, aggregation of AuNPs can be induced, resulting in a red-shift of the plasmon peak around 650-700 nm. | |
| Therefore, the absorption peak at 650 nm is less useful in this approach due to the potential overlap of the nanoparticle aggregation peak. This finding provides important insight into the peroxidase-mimicking activity of AuNPs. | |
| Using this approach, results can be obtained within 90 minutes without complicated or expensive read-out equipment. The method was applied to milk and urine analysis showing promise as a food safety and disease screening tool. | |
| Importantly, coating the AuNP surface with different molecules has the potential to expand the detection capabilities beyond that of proteolytic enzymes. | |
|
Provided by the Institute for Global Food Security, School of Biological Sciences, Queen’s University Belfast, as a Nanowerk exclusive
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
