| Posted: Oct 06, 2006 | |
A new promising class of non-precious metal catalysts for fuel cells |
|
| (Nanowerk Spotlight) Fuel cells are electrochemical energy conversion devices for the direct conversion of the chemical energy of a fuel into electricity. They are among the key enabling technologies for the transition to a hydrogen-based economy. Of several different types of fuel cells under development today, polymer electrolyte fuel cells (PEFCs) have been recognized as a potential future power source for zero emission vehicles. However, to become commercially viable, PEFCs have to overcome the barrier of high catalyst cost caused by the exclusive use of platinum and platinum-based catalysts in the fuel-cell electrodes. Researchers at Los Alamos National Laboratory now demonstrate a new class of low cost (non-precious metal/heteroatomic polymer) nanocomposite catalysts for the PEFC cathode, capable of combining high oxygen-reduction activity with good performance durability. The results of their study show that heteroatomic polymers can be used not only to stabilize the nonprecious metal in the acidic environment of the PEFC cathode but also to generate active sites for oxygen reduction reaction. | |
| Two approaches are at present gaining momentum to replace platinum, which is scarce and expensive. One approach uses non-platinum catalysts that nonetheless contain precious metals with limited abundance and/or limited world distribution. Such catalysts are typically based on palladium or ruthenium. Although platinum is thus avoided, the result is the replacement of one precious metal with another that is on the whole less active than platinum. | |
| An alternative approach is to replace platinum with abundant, nonprecious materials that are not susceptible to price inflation under high-demand circumstances. | |
| The class of non-precious cathode catalysts that has attracted the most attention over the years is pyrolized metal porphyrins, with cobalt and iron porphyrins viewed as the most promising precursors. | |
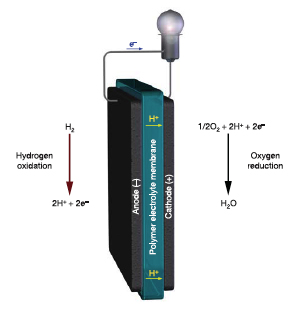 |
Schematic representation of the principle of Polymer electrolyte H2-O2 fuel cell operation (Reprinted with permission from Nature Publishing Group) |
| "The search for practically viable non-precious metal oxygen reduction reaction (ORR) catalysts for the polymer electrolyte fuel cell (PEFC) cathode is by no means new, dating back to 1960's or even before that" Dr. Piotr Zelenay, a Project Leader in the Materials Science and Technology Division of Los Alamos National Laboratory, points out to Nanowerk. "Several different classes of non-precious metal catalysts have been investigated over the years, including pyrolized transition-metal porphyrins. Some of these materials have shown respectable oxygen-reduction activity - still far lower than that of platinum, which is the state-of-the-art ORR (oxygen reduction reaction) catalyst - but not a good stability. Typically, catalyst performance degradation begins as soon as the catalyst is introduced into a fuel cell and continues until it is no longer active." | |
| Zelenay and postdoctoral fellow Rajesh Bashyam describe their new low-cost nanocomposite catalysts in a recent paper, titled "A class of non-precious metal composite catalysts for fuel cells", which appeared in the September 7, 2006 edition of Nature. | |
| "To the best of our knowledge, composite catalysts described in the Nature letter show performance durability that has never been demonstrated with non-precious ORR catalysts" Zelenay says. | |
| The Los Alamos researchers demonstrated that transition-metal oxygen reduction catalysts or, more precisely, an active catalytic centers made of such metals, which are otherwise chemically unstable in the very acidic environment of the PEFC, can be made stable in a heterocyclic-polymer matrix. | |
| The primary potential applications include hydrogen-air PEFCs for automotive transportation and residential power, i.e. relatively high-power fuel-cell based devices. Cost is a main commercialization barrier for such devices, thus making large-scale use of platinum-based catalysts questionable and any non-precious alternatives highly attractive. However, there are still considerable challenges to overcome. | |
| "As in the case of all non-precious metal catalysts, specific activity of the composite catalysts developed by us at Los Alamos is too low to be practical" says Zelenay. "Operating potential of catalysts in this class of materials needs to be raised by at least 0.2 V, if they are to replace platinum in real-life fuel cells." | |
| "In addition to stabilizing non-precious metal catalysts, the key challenge for the kind of electrocatalysis research we have pursued at Los Alamos is to overcome the activity barrier for non-precious metal catalysts. There are several approaches one could take, either separately or in combination. Our approach is to (i) further optimize the composition and structure of the composite materials in the class described in the Nature letter and (ii) modify the ORR electrode (cathode) in fuel cells to allow for higher amount ("loading") of non-precious metal catalysts, that way "making up" for their relatively poor activity." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com.
