Showing Spotlights 81 - 88 of 137 in category All (newest first):
 There is a general perception that nanotechnologies will have a significant impact on developing 'green' and 'clean' technologies with considerable environmental benefits - be it in areas ranging from water treatment to energy breakthroughs and hydrogen applications. As a matter of fact, renewable energy applications probably are the areas where nanotechnology will make its first large-scale commercial breakthroughs. Conflicting with this positive message is the growing body of research that raises questions about the potentially negative effects of engineered nanoparticles on human health and the environment. However, there is one area of nanotechnology that so far hasn't received the necessary attention: the actual processes of manufacturing nanomaterials and the environmental footprint they create, in absolute terms and in comparison with existing industrial manufacturing processes. Analogous to other industrial manufacturing processes, nanoproducts must proceed through various manufacturing stages to produce a material or device with nanoscale dimensions.
There is a general perception that nanotechnologies will have a significant impact on developing 'green' and 'clean' technologies with considerable environmental benefits - be it in areas ranging from water treatment to energy breakthroughs and hydrogen applications. As a matter of fact, renewable energy applications probably are the areas where nanotechnology will make its first large-scale commercial breakthroughs. Conflicting with this positive message is the growing body of research that raises questions about the potentially negative effects of engineered nanoparticles on human health and the environment. However, there is one area of nanotechnology that so far hasn't received the necessary attention: the actual processes of manufacturing nanomaterials and the environmental footprint they create, in absolute terms and in comparison with existing industrial manufacturing processes. Analogous to other industrial manufacturing processes, nanoproducts must proceed through various manufacturing stages to produce a material or device with nanoscale dimensions.
Oct 22nd, 2008
 The way things stand now, nanotechnology products can be sold unlabeled and the FDA regulates sunscreens only based on their sun protection factor. Cosmetic manufacturers, of course, claim that their products, including nanoparticle-based sunscreens are harmless. Indeed, nobody has demonstrated that they are unsafe - but the opposite proof, that they are perfectly safe, is missing as well. This confusing situation is due to the incomplete scientific picture created by a lack of relevant research. For instance, the question of whether or not nanoparticles can penetrate the healthy stratum corneum skin barrier in vivo remains largely unanswered. Furthermore, no studies so far have examined the impact of ultraviolet radiation on nanoparticle skin penetration. Since sunscreen is often applied to sun damaged skin, such a real world scenario, as opposed to in vitro studies in a test-tube, could go a long way in confirming or allaying fears. New research by scientists at the University of Rochester is the first to consider the effects of nanoparticle penetration through normal and barrier defective skin using an in vivo model system.
The way things stand now, nanotechnology products can be sold unlabeled and the FDA regulates sunscreens only based on their sun protection factor. Cosmetic manufacturers, of course, claim that their products, including nanoparticle-based sunscreens are harmless. Indeed, nobody has demonstrated that they are unsafe - but the opposite proof, that they are perfectly safe, is missing as well. This confusing situation is due to the incomplete scientific picture created by a lack of relevant research. For instance, the question of whether or not nanoparticles can penetrate the healthy stratum corneum skin barrier in vivo remains largely unanswered. Furthermore, no studies so far have examined the impact of ultraviolet radiation on nanoparticle skin penetration. Since sunscreen is often applied to sun damaged skin, such a real world scenario, as opposed to in vitro studies in a test-tube, could go a long way in confirming or allaying fears. New research by scientists at the University of Rochester is the first to consider the effects of nanoparticle penetration through normal and barrier defective skin using an in vivo model system.
Aug 20th, 2008
 One term you hear quite often in discussion about the potential risks of nanotechnology is 'precautionary principle'. This moral and political principle, as commonly defined, states that if an action or policy might cause severe or irreversible harm to the public or to the environment, in the absence of a scientific consensus that harm would not ensue, the burden of proof falls on those who would advocate taking the action. The principle aims to provide guidance for protecting public health and the environment in the face of uncertain risks, stating that the absence of full scientific certainty shall not be used as a reason to postpone measures where there is a risk of serious or irreversible harm to public health or the environment. In 2001, an expert panel commissioned by the European Environment Agency (EEA) published a report, Late Lessons from Early Warnings: The Precautionary Principle 1896-2000, which explored 14 case studies, all of which demonstrated how not heeding early warnings had led to a failure to protect human health and the environment. It looked at controversial topics such as asbestos, Mad Cow Disease, growth hormones, PCBs and radiation - all of which demonstrated how not heeding early warnings had led to a failure to protect human health and the environment. The expert group that compiled the EEA report identified 12 'late lessons' on how to avoid past mistakes as new technologies are developed. These lessons bear an uncanny resemblance to many of the concerns now being raised about various forms of nanotechnology.
One term you hear quite often in discussion about the potential risks of nanotechnology is 'precautionary principle'. This moral and political principle, as commonly defined, states that if an action or policy might cause severe or irreversible harm to the public or to the environment, in the absence of a scientific consensus that harm would not ensue, the burden of proof falls on those who would advocate taking the action. The principle aims to provide guidance for protecting public health and the environment in the face of uncertain risks, stating that the absence of full scientific certainty shall not be used as a reason to postpone measures where there is a risk of serious or irreversible harm to public health or the environment. In 2001, an expert panel commissioned by the European Environment Agency (EEA) published a report, Late Lessons from Early Warnings: The Precautionary Principle 1896-2000, which explored 14 case studies, all of which demonstrated how not heeding early warnings had led to a failure to protect human health and the environment. It looked at controversial topics such as asbestos, Mad Cow Disease, growth hormones, PCBs and radiation - all of which demonstrated how not heeding early warnings had led to a failure to protect human health and the environment. The expert group that compiled the EEA report identified 12 'late lessons' on how to avoid past mistakes as new technologies are developed. These lessons bear an uncanny resemblance to many of the concerns now being raised about various forms of nanotechnology.
Jul 22nd, 2008
 Any drug intended for systemic administration and all medical devices which will contact blood must undergo thorough biocompatibility testing. These tests include an in vitro assay to determine the material's potential to damage red blood cells (hemolysis). Hemolysis, the abnormal breakdown of red blood cells either in the blood vessels (intravascular hemolysis) or elsewhere in the body (extravascular), can lead to anemia or other pathological conditions. In the pharmaceutical industry, hematocompatibility testing is harmonized through the use of internationally recognized standard protocols. Nanotechnology- based medical devices and drug carriers are emerging as alternatives to conventional small-molecule drugs, and in vitro evaluation of their biocompatibility with blood components is a necessary part of early preclinical development. Many research papers have reported nanoparticle hemolytic properties but, so far, no in vitro hemolysis protocol has been available that is specific to nanoparticles. A new study published this month describes in vitro assays to study nanoparticle hemolytic properties, identifies nanoparticle interferences with these in vitro tests and provides the first comprehensive insight to potential sources of this interference, demonstrates the usefulness of including nanoparticle-only controls, and illustrates the importance of physicochemical characterization of nanoparticle formulations and visually monitoring test samples to avoid false-positive or false-negative results.
Any drug intended for systemic administration and all medical devices which will contact blood must undergo thorough biocompatibility testing. These tests include an in vitro assay to determine the material's potential to damage red blood cells (hemolysis). Hemolysis, the abnormal breakdown of red blood cells either in the blood vessels (intravascular hemolysis) or elsewhere in the body (extravascular), can lead to anemia or other pathological conditions. In the pharmaceutical industry, hematocompatibility testing is harmonized through the use of internationally recognized standard protocols. Nanotechnology- based medical devices and drug carriers are emerging as alternatives to conventional small-molecule drugs, and in vitro evaluation of their biocompatibility with blood components is a necessary part of early preclinical development. Many research papers have reported nanoparticle hemolytic properties but, so far, no in vitro hemolysis protocol has been available that is specific to nanoparticles. A new study published this month describes in vitro assays to study nanoparticle hemolytic properties, identifies nanoparticle interferences with these in vitro tests and provides the first comprehensive insight to potential sources of this interference, demonstrates the usefulness of including nanoparticle-only controls, and illustrates the importance of physicochemical characterization of nanoparticle formulations and visually monitoring test samples to avoid false-positive or false-negative results.
Jul 18th, 2008
 Nanoparticles with at least one dimension of 100 nanometers or less fall in the transitional zone between individual atoms or molecules and the corresponding bulk material, which can drastically modify the physicochemical properties of the material and may generate adverse biological effects in organisms. As the discussion about potentially undesired side effects of engineered nanoparticles heats up, research on toxicological effects of nanomaterials gets increasing attention. Nanotoxicology is quickly being established as a new field, with its major focus on human and animal studies. However, very few studies have been conducted to assess the toxicity of nanomaterials to ecological terrestrial species, particularly plants. So far, the mechanisms of nanoparticle phytotoxicity ? the ability to cause injury to plants ? remain largely unknown and little information on the potential uptake of nanoparticles by plants and their subsequent fate within the food chain is available.
Nanoparticles with at least one dimension of 100 nanometers or less fall in the transitional zone between individual atoms or molecules and the corresponding bulk material, which can drastically modify the physicochemical properties of the material and may generate adverse biological effects in organisms. As the discussion about potentially undesired side effects of engineered nanoparticles heats up, research on toxicological effects of nanomaterials gets increasing attention. Nanotoxicology is quickly being established as a new field, with its major focus on human and animal studies. However, very few studies have been conducted to assess the toxicity of nanomaterials to ecological terrestrial species, particularly plants. So far, the mechanisms of nanoparticle phytotoxicity ? the ability to cause injury to plants ? remain largely unknown and little information on the potential uptake of nanoparticles by plants and their subsequent fate within the food chain is available.
Jul 9th, 2008
 Engineered nanoparticles are rapidly becoming a part of our daily life in the form of cosmetics, food packaging, drug delivery systems, therapeutics, biosensors, etc. A number of commercial products such as wound dressing, detergents or antimicrobial coatings are already in the market. Although little is known about their bio distribution and bio activity, especially silver nanoparticles are extensively used for all kinds of antimicrobial applications. Ultimately, these nanoparticles end up in the environment during waste disposal. Largely due to a scarcity of data on the toxicity, intracellular distribution and fate of silver ions and nanoparticles inside an organism, regulatory bodies so far have not felt the need to regulate the use of such materials in commercial products or disposal of such products. In order to improve the scientific data and to enhance our insight on the health and environmental impact of silver nanoparticles, scientists in Singapore have initiated an in vivo toxicology study to examine nanosilver in a zebrafish model. They conclude that silver nanoparticles have the potential to cause health and ecotoxicity issues in a concentration-dependent manner.
Engineered nanoparticles are rapidly becoming a part of our daily life in the form of cosmetics, food packaging, drug delivery systems, therapeutics, biosensors, etc. A number of commercial products such as wound dressing, detergents or antimicrobial coatings are already in the market. Although little is known about their bio distribution and bio activity, especially silver nanoparticles are extensively used for all kinds of antimicrobial applications. Ultimately, these nanoparticles end up in the environment during waste disposal. Largely due to a scarcity of data on the toxicity, intracellular distribution and fate of silver ions and nanoparticles inside an organism, regulatory bodies so far have not felt the need to regulate the use of such materials in commercial products or disposal of such products. In order to improve the scientific data and to enhance our insight on the health and environmental impact of silver nanoparticles, scientists in Singapore have initiated an in vivo toxicology study to examine nanosilver in a zebrafish model. They conclude that silver nanoparticles have the potential to cause health and ecotoxicity issues in a concentration-dependent manner.
Jun 6th, 2008
 Germany, with an almost 40% share of European public funded nanoscience research, is the clear nanotechnology leader in Europe. It is also one of the leaders globally in pushing research into potential risk and safety concerns associated with nanotechnology. The Federal Ministry of Education and Research (BMBF) is the ministry responsible for federal activities in the nanotechnology sector in Germany. Within its framework of 'leading-edge innovations' the BMBF supports key areas of nanotechnologies with promising prospects (NanoMobil, NanoChem, NanoFab, NanoforLife,NanoLux). The project NanoChance aims to support small and medium-sized companies in particular. The cooperative project NanoCare currently mainly focuses on studying possible risks of engineered nanoparticles. Beyond that, the federal agencies BAuA (Federal Institute for Occupational Safety and Health), UBA (Federal Environment Agency) and BfR (Federal Institute for Risk Assessment) have developed a joint research strategy that addresses especially health and environmental risks of engineered nanoparticles. The strategy has been finalized in December 2007 and a final report has just been published.
Germany, with an almost 40% share of European public funded nanoscience research, is the clear nanotechnology leader in Europe. It is also one of the leaders globally in pushing research into potential risk and safety concerns associated with nanotechnology. The Federal Ministry of Education and Research (BMBF) is the ministry responsible for federal activities in the nanotechnology sector in Germany. Within its framework of 'leading-edge innovations' the BMBF supports key areas of nanotechnologies with promising prospects (NanoMobil, NanoChem, NanoFab, NanoforLife,NanoLux). The project NanoChance aims to support small and medium-sized companies in particular. The cooperative project NanoCare currently mainly focuses on studying possible risks of engineered nanoparticles. Beyond that, the federal agencies BAuA (Federal Institute for Occupational Safety and Health), UBA (Federal Environment Agency) and BfR (Federal Institute for Risk Assessment) have developed a joint research strategy that addresses especially health and environmental risks of engineered nanoparticles. The strategy has been finalized in December 2007 and a final report has just been published.
May 29th, 2008
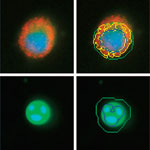 High content analysis (HCA) is a powerful platform that combines cell-based assays with traditional microscopy and automated, sophisticated image processing and analysis software. This technology is capable of using living and fixed cells, typically with fluorescently labeled antibodies and reagents. It has been widely adopted in the pharmaceutical and biotechnology industries for target identification and validation. HCA has made particular inroads into research and development applications where high throughput screening has proven inadequate, such as measuring multiple biological pathways simultaneously, or revealing off-target drug effects. HCA has stepped into this void by demonstrating how particular proteins are affected by the application of a molecule to the cell line of interest.
High content analysis (HCA) is a powerful platform that combines cell-based assays with traditional microscopy and automated, sophisticated image processing and analysis software. This technology is capable of using living and fixed cells, typically with fluorescently labeled antibodies and reagents. It has been widely adopted in the pharmaceutical and biotechnology industries for target identification and validation. HCA has made particular inroads into research and development applications where high throughput screening has proven inadequate, such as measuring multiple biological pathways simultaneously, or revealing off-target drug effects. HCA has stepped into this void by demonstrating how particular proteins are affected by the application of a molecule to the cell line of interest.
May 20th, 2008
 There is a general perception that nanotechnologies will have a significant impact on developing 'green' and 'clean' technologies with considerable environmental benefits - be it in areas ranging from water treatment to energy breakthroughs and hydrogen applications. As a matter of fact, renewable energy applications probably are the areas where nanotechnology will make its first large-scale commercial breakthroughs. Conflicting with this positive message is the growing body of research that raises questions about the potentially negative effects of engineered nanoparticles on human health and the environment. However, there is one area of nanotechnology that so far hasn't received the necessary attention: the actual processes of manufacturing nanomaterials and the environmental footprint they create, in absolute terms and in comparison with existing industrial manufacturing processes. Analogous to other industrial manufacturing processes, nanoproducts must proceed through various manufacturing stages to produce a material or device with nanoscale dimensions.
There is a general perception that nanotechnologies will have a significant impact on developing 'green' and 'clean' technologies with considerable environmental benefits - be it in areas ranging from water treatment to energy breakthroughs and hydrogen applications. As a matter of fact, renewable energy applications probably are the areas where nanotechnology will make its first large-scale commercial breakthroughs. Conflicting with this positive message is the growing body of research that raises questions about the potentially negative effects of engineered nanoparticles on human health and the environment. However, there is one area of nanotechnology that so far hasn't received the necessary attention: the actual processes of manufacturing nanomaterials and the environmental footprint they create, in absolute terms and in comparison with existing industrial manufacturing processes. Analogous to other industrial manufacturing processes, nanoproducts must proceed through various manufacturing stages to produce a material or device with nanoscale dimensions. 
 Subscribe to our Nanotechnology Spotlight feed
Subscribe to our Nanotechnology Spotlight feed





