Showing Spotlights 57 - 64 of 137 in category All (newest first):
 The discussion about nanotechnology related safety issues so far has focused mainly on three areas - consumers getting exposed to products containing nanomaterials; nanomaterials getting released into the environment and potentially entering the food chain; and industrial workers being exposed to nanomaterials during the production process. There is an increasing number of reports and research papers dealing with these issues. Interestingly, while surveys of nanotechnology safety practices have concentrated on industrial settings, the safety issues of a significant number of people working with nanomaterials have not been addressed in a concerted matter - the researchers at university and private research laboratories who are doing all the early stage research and development. According to a survey conducted by a Spanish research group, it appears that the nanotechnology research community is not exactly at the forefront when it comes to following, not to mention setting, standards for safe practices for handling nanomaterials.
The discussion about nanotechnology related safety issues so far has focused mainly on three areas - consumers getting exposed to products containing nanomaterials; nanomaterials getting released into the environment and potentially entering the food chain; and industrial workers being exposed to nanomaterials during the production process. There is an increasing number of reports and research papers dealing with these issues. Interestingly, while surveys of nanotechnology safety practices have concentrated on industrial settings, the safety issues of a significant number of people working with nanomaterials have not been addressed in a concerted matter - the researchers at university and private research laboratories who are doing all the early stage research and development. According to a survey conducted by a Spanish research group, it appears that the nanotechnology research community is not exactly at the forefront when it comes to following, not to mention setting, standards for safe practices for handling nanomaterials.
Feb 11th, 2010
 In a nanotechnology risk assessment study published last year, researchers concluded that the costs associated with nanomaterial risk assessment in the United States alone could range anywhere from $249 million to $1.18 billion and might take decades to complete at current levels of investment in nano-hazard testing. While research in quantitative risk characterization of nanomaterials is crucially important, and no one advocates abandoning this approach, scientists and policy makers must face the reality that many of these knowledge gaps cannot be expected to be closed for many years to come - and decision making will need to continue under conditions of uncertainty. At the same time, current chemical-based research efforts are mainly directed at establishing toxicological and ecotoxicological and exposure data for nanomaterials, with comparatively little research undertaken on the tools or approaches that may facilitate near-term decisions. A group of scientists suggests that this situation requires a significant research program in a fundamental area of timely, yet informed decision making regarding the potential risks of nanomaterials. They highlight some of these issues as well as outline some of the currently available tools and approaches for decision making regarding the potential risks of nanomaterials.
In a nanotechnology risk assessment study published last year, researchers concluded that the costs associated with nanomaterial risk assessment in the United States alone could range anywhere from $249 million to $1.18 billion and might take decades to complete at current levels of investment in nano-hazard testing. While research in quantitative risk characterization of nanomaterials is crucially important, and no one advocates abandoning this approach, scientists and policy makers must face the reality that many of these knowledge gaps cannot be expected to be closed for many years to come - and decision making will need to continue under conditions of uncertainty. At the same time, current chemical-based research efforts are mainly directed at establishing toxicological and ecotoxicological and exposure data for nanomaterials, with comparatively little research undertaken on the tools or approaches that may facilitate near-term decisions. A group of scientists suggests that this situation requires a significant research program in a fundamental area of timely, yet informed decision making regarding the potential risks of nanomaterials. They highlight some of these issues as well as outline some of the currently available tools and approaches for decision making regarding the potential risks of nanomaterials.
Jan 20th, 2010
 Experts and the public generally differ in their perceptions of technology risk. While this might be due to social and demographic factors, it is generally assumed by scientists who conduct risk research that experts' risk assessments are based more strongly on actual or perceived knowledge about a technology than lay people's risk assessments. Nevertheless, whether the risks are real or not, the public perception of an emerging technology will have a major influence on the acceptance of this technology and its commercial success. If the public perception turns negative, potentially beneficial technologies will be severely constrained as is the case for instance with gene technology. It is not surprising that a new study found that, in general, nanoscientists are more optimistic than the public about the potential benefits of nanotechnology. What is surprising though, is that, for some issues related to the environmental and long-term health impacts of nanotechnology, nanoscientists seem to be significantly more concerned than the public. Arguing that risk communication on nanotechnologies requires target-specific approaches, a group of researchers in Germany advocate the development of communication strategies that help people to comprehend nanotechnology, to differentiate between the fields of application and to gain an understanding of the cause and effect chains.
Experts and the public generally differ in their perceptions of technology risk. While this might be due to social and demographic factors, it is generally assumed by scientists who conduct risk research that experts' risk assessments are based more strongly on actual or perceived knowledge about a technology than lay people's risk assessments. Nevertheless, whether the risks are real or not, the public perception of an emerging technology will have a major influence on the acceptance of this technology and its commercial success. If the public perception turns negative, potentially beneficial technologies will be severely constrained as is the case for instance with gene technology. It is not surprising that a new study found that, in general, nanoscientists are more optimistic than the public about the potential benefits of nanotechnology. What is surprising though, is that, for some issues related to the environmental and long-term health impacts of nanotechnology, nanoscientists seem to be significantly more concerned than the public. Arguing that risk communication on nanotechnologies requires target-specific approaches, a group of researchers in Germany advocate the development of communication strategies that help people to comprehend nanotechnology, to differentiate between the fields of application and to gain an understanding of the cause and effect chains.
Jan 14th, 2010
 A scarcity of empirical data - especially regarding losses - hampers nanotechnology-related risk dialogue. Nanotechnology is a growing niche, so there is little litigation or loss history to analyze. Thus, much of the discussion of nanotechnology and its management flows from hypothetical examples. Less murky is the fact that nanotechnology is not a passing fad. It has innovative applications for a range of technologies and sectors, including drug delivery, medical imaging, integrated sensors, and semiconductors. The biggest areas of nanotechnology risk management concerns lies in workers' compensation and product liability. This article looks at industry responses and risk management strategies.
A scarcity of empirical data - especially regarding losses - hampers nanotechnology-related risk dialogue. Nanotechnology is a growing niche, so there is little litigation or loss history to analyze. Thus, much of the discussion of nanotechnology and its management flows from hypothetical examples. Less murky is the fact that nanotechnology is not a passing fad. It has innovative applications for a range of technologies and sectors, including drug delivery, medical imaging, integrated sensors, and semiconductors. The biggest areas of nanotechnology risk management concerns lies in workers' compensation and product liability. This article looks at industry responses and risk management strategies.
Dec 15th, 2009
 A newly published antibacterial activity mechanism study demonstrates how a single walled carbon nanotube (SWCNT) kills bacteria by the physical puncture of bacterial membranes. The nanotubes would constantly attack the bacteria in solution, degrading the bacterial cell integrity and causing the cell death. This work elucidates several factors controlling the antibacterial activity of pristine SWCNTs and provides an insight in their toxicity mechanism. With regard to carbon nanotubes, in early toxicological studies, researchers obtained confounding results - in some studies nanotubes were toxic; in others, they were not. The apparent contradictions were actually a result of the materials that the researchers were using.
A newly published antibacterial activity mechanism study demonstrates how a single walled carbon nanotube (SWCNT) kills bacteria by the physical puncture of bacterial membranes. The nanotubes would constantly attack the bacteria in solution, degrading the bacterial cell integrity and causing the cell death. This work elucidates several factors controlling the antibacterial activity of pristine SWCNTs and provides an insight in their toxicity mechanism. With regard to carbon nanotubes, in early toxicological studies, researchers obtained confounding results - in some studies nanotubes were toxic; in others, they were not. The apparent contradictions were actually a result of the materials that the researchers were using.
Nov 10th, 2009
 The potential use of antimicrobial surface coatings ranges from medicine, where medical device infection is associated with significant healthcare costs, to the construction industry and the food packaging industry. Thin films which contain silver have been seen as promising candidate coatings. There now are even anti-odor, anti-bacterial socks that are treated with silver nanoparticles. Researchers in Switzerland have now examined what happens to these silver nanoparticle-treated textiles during washing. The scientists studied release of nanoparticles in laundry water from nine different textiles, including different brands of commercially available anti-odor socks. Studies like these will help address the question what the chances are of nanoparticles from nanofinished textiles being released into the environment.
The potential use of antimicrobial surface coatings ranges from medicine, where medical device infection is associated with significant healthcare costs, to the construction industry and the food packaging industry. Thin films which contain silver have been seen as promising candidate coatings. There now are even anti-odor, anti-bacterial socks that are treated with silver nanoparticles. Researchers in Switzerland have now examined what happens to these silver nanoparticle-treated textiles during washing. The scientists studied release of nanoparticles in laundry water from nine different textiles, including different brands of commercially available anti-odor socks. Studies like these will help address the question what the chances are of nanoparticles from nanofinished textiles being released into the environment.
Nov 4th, 2009
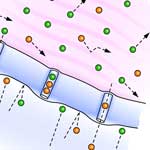 The interest in exploring the use of noble metal nanoparticles for diagnostic and therapeutic imaging stems from the drawbacks of current in vivo probes. Fluorescent probes, such as fluorescent dyes and proteins, are not photostable and therefore are useful only for a limited time during the probing event. Besides imaging agents, especially gold nanoparticles are also intensely researched as target-specific vehicles for drug delivery. Due to its inert chemical properties, gold has been widely considered as one of the most stable and biocompatible materials. But studies of the biocompatibility and toxicity of gold nanoparticles in various types of cells have yielded inconclusive results: some studies show a toxic effect and high-dependence of toxicity on nanoparticle size and surface functional groups, while other studies report no significant cytotoxicity. Many of these studies did not use purified gold nanoparticles, or examine any other chemicals present in the gold nanoparticle solutions, or well characterize the physical properties, leading to these inconclusive results. Researchers have now synthesized and characterized stable, nearly monodisperse, and highly purified gold nanoparticles, and utilized them to study cleavage-stage embryos in real-time and to probe their effects on embryonic development at the single-nanoparticle level in real time.
The interest in exploring the use of noble metal nanoparticles for diagnostic and therapeutic imaging stems from the drawbacks of current in vivo probes. Fluorescent probes, such as fluorescent dyes and proteins, are not photostable and therefore are useful only for a limited time during the probing event. Besides imaging agents, especially gold nanoparticles are also intensely researched as target-specific vehicles for drug delivery. Due to its inert chemical properties, gold has been widely considered as one of the most stable and biocompatible materials. But studies of the biocompatibility and toxicity of gold nanoparticles in various types of cells have yielded inconclusive results: some studies show a toxic effect and high-dependence of toxicity on nanoparticle size and surface functional groups, while other studies report no significant cytotoxicity. Many of these studies did not use purified gold nanoparticles, or examine any other chemicals present in the gold nanoparticle solutions, or well characterize the physical properties, leading to these inconclusive results. Researchers have now synthesized and characterized stable, nearly monodisperse, and highly purified gold nanoparticles, and utilized them to study cleavage-stage embryos in real-time and to probe their effects on embryonic development at the single-nanoparticle level in real time.
Sep 11th, 2009
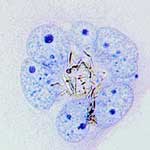 One of the complications of nanotoxicology is that the toxicity of a specific nanomaterial cannot be predicted from the toxicity of the same material in a different form. For instance, while the toxicity of inert systems such as iron oxides, gold, or silver has been investigated for nearly isotropic particles, the toxicity of these materials in nanofilament form cannot be predicted from their known toxicity as nanoparticles. Fully understanding the toxic mechanisms of nanoscale materials is an essential prerequisite in being able to design harmless nanomaterials whose interactions with biological cells is non-lethal. Currently, a lot of nanotoxicological research effort is focused on carbon nanotubes, but nanofilaments are not exclusively based on carbon materials and can be produced from many inorganic materials in the form of nanotubes and nanowires. Applying the 'precautionary principle' to nanotechnology would require much more extensive nanotoxicological research on all types of nanomaterials; and there seems to be a particular lack of findings concerning non-carbon nanofilaments. Researchers in Switzerland have now taken a closer look at the fate of titanium dioxide (TiO2) based nanofilaments in the body. Their results are cause for concern.
One of the complications of nanotoxicology is that the toxicity of a specific nanomaterial cannot be predicted from the toxicity of the same material in a different form. For instance, while the toxicity of inert systems such as iron oxides, gold, or silver has been investigated for nearly isotropic particles, the toxicity of these materials in nanofilament form cannot be predicted from their known toxicity as nanoparticles. Fully understanding the toxic mechanisms of nanoscale materials is an essential prerequisite in being able to design harmless nanomaterials whose interactions with biological cells is non-lethal. Currently, a lot of nanotoxicological research effort is focused on carbon nanotubes, but nanofilaments are not exclusively based on carbon materials and can be produced from many inorganic materials in the form of nanotubes and nanowires. Applying the 'precautionary principle' to nanotechnology would require much more extensive nanotoxicological research on all types of nanomaterials; and there seems to be a particular lack of findings concerning non-carbon nanofilaments. Researchers in Switzerland have now taken a closer look at the fate of titanium dioxide (TiO2) based nanofilaments in the body. Their results are cause for concern.
Jul 27th, 2009
 The discussion about nanotechnology related safety issues so far has focused mainly on three areas - consumers getting exposed to products containing nanomaterials; nanomaterials getting released into the environment and potentially entering the food chain; and industrial workers being exposed to nanomaterials during the production process. There is an increasing number of reports and research papers dealing with these issues. Interestingly, while surveys of nanotechnology safety practices have concentrated on industrial settings, the safety issues of a significant number of people working with nanomaterials have not been addressed in a concerted matter - the researchers at university and private research laboratories who are doing all the early stage research and development. According to a survey conducted by a Spanish research group, it appears that the nanotechnology research community is not exactly at the forefront when it comes to following, not to mention setting, standards for safe practices for handling nanomaterials.
The discussion about nanotechnology related safety issues so far has focused mainly on three areas - consumers getting exposed to products containing nanomaterials; nanomaterials getting released into the environment and potentially entering the food chain; and industrial workers being exposed to nanomaterials during the production process. There is an increasing number of reports and research papers dealing with these issues. Interestingly, while surveys of nanotechnology safety practices have concentrated on industrial settings, the safety issues of a significant number of people working with nanomaterials have not been addressed in a concerted matter - the researchers at university and private research laboratories who are doing all the early stage research and development. According to a survey conducted by a Spanish research group, it appears that the nanotechnology research community is not exactly at the forefront when it comes to following, not to mention setting, standards for safe practices for handling nanomaterials.
 Subscribe to our Nanotechnology Spotlight feed
Subscribe to our Nanotechnology Spotlight feed





