Showing Spotlights 105 - 112 of 333 in category All (newest first):
 An international team of researchers has demonstrated a simple method for transferring A4-size sheets of CVD graphene from copper foils onto a target substrate using a commercially available polyvinyl alcohol (PVA) polymer foil as a carrier substrate and an off-the-shelf office laminator. This do-it-yourself approach requires few tools and low cost materials - it is safe and easy enough to be carried out in school physics classes. There are no chemicals involved besides water; no spinner; no dangerous etchants.
An international team of researchers has demonstrated a simple method for transferring A4-size sheets of CVD graphene from copper foils onto a target substrate using a commercially available polyvinyl alcohol (PVA) polymer foil as a carrier substrate and an off-the-shelf office laminator. This do-it-yourself approach requires few tools and low cost materials - it is safe and easy enough to be carried out in school physics classes. There are no chemicals involved besides water; no spinner; no dangerous etchants.
Mar 25th, 2019
 Alpha-synuclein is a protein whose function in the healthy brain is currently unknown. It is of great interest to Parkinson's researchers because it is a major constituent of Lewy bodies, protein clumps that are the pathological hallmark of Parkinson's disease (PD). Scientists believe that the self-assembly of alpha-synuclein into oligomers and fibrils is linked to progress and pathogenesis of the disease. A new study suggest that important characteristics of the fibrillation process, such as surface charge and surface functional group, should be considered in the development of nanotechnology-based therapeutic approaches.
Alpha-synuclein is a protein whose function in the healthy brain is currently unknown. It is of great interest to Parkinson's researchers because it is a major constituent of Lewy bodies, protein clumps that are the pathological hallmark of Parkinson's disease (PD). Scientists believe that the self-assembly of alpha-synuclein into oligomers and fibrils is linked to progress and pathogenesis of the disease. A new study suggest that important characteristics of the fibrillation process, such as surface charge and surface functional group, should be considered in the development of nanotechnology-based therapeutic approaches.
Mar 19th, 2019
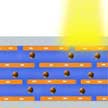 Recent research in nanofluidics has adopted reconstructed layered two-dimensional (2D) sheets as a promising material platform for nanofluidics. These membranes contain a high volume fraction of interconnected 2D nanochannels. In new work, researchers demonstrate a coupled photon-electron-ion transport phenomenon through graphene oxide membranes. It shows a straightforward way on how to power the transport in 2D layered materials using the energy of light.
Recent research in nanofluidics has adopted reconstructed layered two-dimensional (2D) sheets as a promising material platform for nanofluidics. These membranes contain a high volume fraction of interconnected 2D nanochannels. In new work, researchers demonstrate a coupled photon-electron-ion transport phenomenon through graphene oxide membranes. It shows a straightforward way on how to power the transport in 2D layered materials using the energy of light.
Mar 18th, 2019
 In recent years, all-optical modulators (AOMs) have attracted significant interests due to their low power consumption, broad bandwidth, and potential in all-optical fields. Among these, AOMs based on the high photothermal efficiency of antimonene, exhibit remarkable advantages for their large modulation depth, wide operating wavelength range, and easy implementation.
Researchers demonstrated that an antimonene-based AOM was successfully utilized to actively Q-switch a fiber laser in a fully photonics domain and this actively modulated laser represented all-optically tunable output parameters, and easy time synchronization.
In recent years, all-optical modulators (AOMs) have attracted significant interests due to their low power consumption, broad bandwidth, and potential in all-optical fields. Among these, AOMs based on the high photothermal efficiency of antimonene, exhibit remarkable advantages for their large modulation depth, wide operating wavelength range, and easy implementation.
Researchers demonstrated that an antimonene-based AOM was successfully utilized to actively Q-switch a fiber laser in a fully photonics domain and this actively modulated laser represented all-optically tunable output parameters, and easy time synchronization.
Mar 15th, 2019
 Electronic tattoos (e-tattoos) are an extremely thin form of wearable electronics. They are lightweight and soft, which allows them to be intimately mounted on human skin for noninvasive, high-fidelity sensing. During the operation of e-tattoos, they are constantly exposed to external mechanical inputs such as bending, twisting, pressing, and cutting, which may cause mechanical damage and lead to malfunction. Now, researchers have demonstrated a self-healing silk e-tattoo that shows high sensitivity to multiple stimuli, including strain, humidity, and temperature based on a unique graphene, silk fibroin, Ca2+ combination.
Electronic tattoos (e-tattoos) are an extremely thin form of wearable electronics. They are lightweight and soft, which allows them to be intimately mounted on human skin for noninvasive, high-fidelity sensing. During the operation of e-tattoos, they are constantly exposed to external mechanical inputs such as bending, twisting, pressing, and cutting, which may cause mechanical damage and lead to malfunction. Now, researchers have demonstrated a self-healing silk e-tattoo that shows high sensitivity to multiple stimuli, including strain, humidity, and temperature based on a unique graphene, silk fibroin, Ca2+ combination.
Mar 8th, 2019
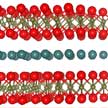 Potassium niobate (KNbO3) is a a perovskite ferroelectric crystal that, due to its optical properties, has been found useful in many different areas of materials science research, including electro-optics, piezoelectric, and electronic applications. Researchers have now, for the first time, succeeded in producing large KNbO3 crystals with uniform flat shape, and c-axis texture, which makes the crystals useful for practical electro-optic applications. By using MXenes as precursor materials to grow ferroelectric crystals, their 2D nature is inherited by the ferroelectric crystals.
Potassium niobate (KNbO3) is a a perovskite ferroelectric crystal that, due to its optical properties, has been found useful in many different areas of materials science research, including electro-optics, piezoelectric, and electronic applications. Researchers have now, for the first time, succeeded in producing large KNbO3 crystals with uniform flat shape, and c-axis texture, which makes the crystals useful for practical electro-optic applications. By using MXenes as precursor materials to grow ferroelectric crystals, their 2D nature is inherited by the ferroelectric crystals.
Feb 25th, 2019
 The formation of dendrites on the metal electrodes of lithium metal batteries causes safety and performance concerns. To avoid dendrites, researchers are experimenting with new battery electrolyte chemistries, new separator technologies, and new physical hosts for the lithium metal. Researchers now have discovered a key design rule for Li metal batteries: If you want to suppress dendrites, you have to use a defect-free host. More generally, carbon defects catalyze dendrite growth in metal anodes.
The formation of dendrites on the metal electrodes of lithium metal batteries causes safety and performance concerns. To avoid dendrites, researchers are experimenting with new battery electrolyte chemistries, new separator technologies, and new physical hosts for the lithium metal. Researchers now have discovered a key design rule for Li metal batteries: If you want to suppress dendrites, you have to use a defect-free host. More generally, carbon defects catalyze dendrite growth in metal anodes.
Feb 13th, 2019
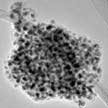 Researchers report significant advances in developing a high-temperature, fast nanomanufacturing technique for the large-scale production of ultra small metal nanoclusters decorated in a graphene host matrix. The size and morphology of the metal nanoclusters can be controlled by varying the reaction temperature and the mass loading of metal salt in the graphene aerosol nanoreactors. This one-step continuous aerosol-based thermal shock technique offers considerable potential for the manufacturing of well-dispersed and uniform nanoclusters stabilized within a host matrix.
Researchers report significant advances in developing a high-temperature, fast nanomanufacturing technique for the large-scale production of ultra small metal nanoclusters decorated in a graphene host matrix. The size and morphology of the metal nanoclusters can be controlled by varying the reaction temperature and the mass loading of metal salt in the graphene aerosol nanoreactors. This one-step continuous aerosol-based thermal shock technique offers considerable potential for the manufacturing of well-dispersed and uniform nanoclusters stabilized within a host matrix.
Feb 4th, 2019
 An international team of researchers has demonstrated a simple method for transferring A4-size sheets of CVD graphene from copper foils onto a target substrate using a commercially available polyvinyl alcohol (PVA) polymer foil as a carrier substrate and an off-the-shelf office laminator. This do-it-yourself approach requires few tools and low cost materials - it is safe and easy enough to be carried out in school physics classes. There are no chemicals involved besides water; no spinner; no dangerous etchants.
An international team of researchers has demonstrated a simple method for transferring A4-size sheets of CVD graphene from copper foils onto a target substrate using a commercially available polyvinyl alcohol (PVA) polymer foil as a carrier substrate and an off-the-shelf office laminator. This do-it-yourself approach requires few tools and low cost materials - it is safe and easy enough to be carried out in school physics classes. There are no chemicals involved besides water; no spinner; no dangerous etchants.
 Subscribe to our Nanotechnology Spotlight feed
Subscribe to our Nanotechnology Spotlight feed





