| Posted: May 29, 2009 | |
Combining the properties of fullerenes and polymers for next-generation solar cells |
|
| (Nanowerk Spotlight) Increasing the efficiencies of polymer-based solar cells while at the same time keeping production complexity and cost low will require the preparation of new classes of polymers that can be prepared with a minimum of synthetic steps. Combining strong electron acceptors such as fullerenes (C60) with commodity polymers to make electronically active polymers promises to be one possible route. So far, though, the photovoltaic efficiencies of polymer/C60 blends are generally not as good as those for photovoltaic devices made from the currently used main classes of polymers, P3HT and PCBM. | |
| "We wanted to find a simple way to simultaneously exploit the expected benefits of polymers and the electron acceptor properties of fullerene," Roger C. Hiorns tells Nanowerk. "This pointed us in the direction of main-chain fullerene polymers. There are examples in the literature, but they generally require multi step syntheses, can be 'invasive' with respect to the electronic structure of the C60 due to the formation of multiple bonds, or result in insoluble products due to crosslinking." | |
| What Hiorns, a scientist at the Laboratoire de Chimie des Polymères Organiques (UMR 5629), Ecole Nationale Supérieure de Chimie et de Physique de Bordeaux and collaborators from various departments at the University of Bordeaux came up with is a way to very simply prepare polymers from fullerenes without having to strongly change the aromaticity of the C60 sphere. This means that many of the original properties of C60 may be found to be retained even when combined with the beneficial properties of polymers. | |
| "Obviously this is early days as this is the first prototype of this sort of material" says Hiorns. "There have been other examples of polymers incorporating fullerenes, but more often than not they require tortuous syntheses, or rupture the C60 aromaticity or end up crosslinking or any combination of these things." | |
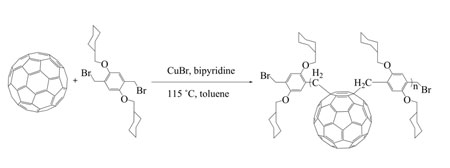
| Hiorns, together with Eric Cloutet, is first author of a recent paper in Macromolecules that describes these findings ("Main-Chain Fullerene Polymers for Photovoltaic Devices"). | |
 |
|
| Proposed synthetic route to PFDP. (Image: Dr. Roger C. Hiorns, Université de Bordeaux) | |
| The new method of synthesis is very simple and results in nicely soluble polymers that contain high degrees of fullerenes. According to Hiorns and Cloutet, there seem to be only two provisos for this reaction: The first is that the groups that join the C60s together should be quite large – that way they block secondary reactions occurring around the C60 and make sure that there is a linear chain at the end of the reaction. The second is that the same groups should carry two alkyl-bromides each so as to join the C60s up through atom transfer radical addition chemistry. | |
| "What is really quite exciting is that the properties of C60 and polymers may be combined" Hiorns points out. "C60 is known for its electronic properties, opto-electronic properties and even its biological activity. We would hope that the incorporation of C60 in to polymeric structures may further its ease of use and vary its properties. This brings another point to mind. You'll notice in the scheme that the C60 alternates with another group. It is possible that by changing the nature of this group, we can vary the properties of these polymers considerably. So really you might say that the best thing about this discovery is that it brings in another route to making new polymers that incorporate C60." | |
| With regard to the photovoltaic properties, the new polymer that the French team have called PFDP (the full name is quite a mouth full: poly{(1,4-fullerene)-alt-[1,4-dimethylene-2,5-bis(cyclohexylmethyl ether)phenylene]}s) shows some extraordinary properties that they don't fully understand yet. | |
| "It seems to form aggregates when mixed and heated in a composite with poly(3-hexylthiophene) (P3HT) that are about 20 nm wide," say Hiorns and Cloutet. "This is useful in an organic solar cell because the charges are collected from excitonic states that form when the composite is irradiated. These states can only 'live' over about 10-20 nm and need an interface between the PFDP and the P3HT to be converted into charges. Once negative and positive charges are formed they move through each phase – probably mostly electrons for the PFDP and mostly holes for the P3HT – to the electrodes. Hence the electricity." | |
| The chemistry for this work was inspired by previous research on alternatively linking fullerene and conjugated polymers, described in a paper in Journal of Polymer Science Part A: Polymer Chemistry, also first-authored by Hiorns. | |
| It appears that this prototype polymer opens up the path to many other new materials with interesting polymeric/electronic/opto-electronic and maybe even biological properties. Hiorns and Cloutet point out that the main problem with this system is finding out how to join the C60s together. If the groups reacting on the C60 are not big enough then there are lots of secondary reactions. So the real problem here will be to find out which groups can insert themselves in between the C60s and keep making the chain linear rather than branched and then insoluble (while it is linear it stays soluble in most organic solvents such as toluene, dichlorobenzene, and so on). | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
