| Posted: Jul 06, 2016 | |
Nanotechnology-based approach to repair the cancer cell suicide mechanism |
|
| (Nanowerk Spotlight) Cancer is a very complex disease and the exact cause is not clearly understood yet. Extensive biomedical research suggests a combination of genetic and environmental factors. Treatment for cancer can take many forms and primarily depends on the type of the disease, its progression and other factors. | |
| Under normal circumstances, apoptosis is a highly regulated and controlled biochemical process that leads to cell death. In other words, this is a natural process by which cells 'commit suicide'. Cancer cells can outsmart and evade this normal cellular mechanism, leading to uncontrolled cellular growth. | |
| Mitochondria are the primary controllers of cellular suicide. Mitochondria are known as the ‘powerhouse of the cell’ and function as a battery to supply energy for the manufacture of ATP (the energy storage molecule adenosine triphosphate). | |
| "In the 1950s, Otto Warburg (Nobel Prize Winner, 1931) proposed a linkage between cancer and functional damage to mitochondria. Although this theory has been long debated, emerging studies have instigated further validation that mitochondrial metabolism theoretically is a plausible target for cancer therapy," Dipanjan Pan, an Assistant Professor in the Department of Bioengineering at the Beckman Institute of Advanced Science and Technology, University of Illinois at Urbana-Champaign, tells Nanowerk. "To trigger the suicide switch back on, our work uses a highly selective nanotechnology-based approach to deliver a widely available small molecule commonly found as a by-product of a water chlorination process." | |
| In a preclinical model, Pan and his team demonstrated remarkable success of these agents. They reported their findings in the June 21, 2016 online edition of Scientific Reports ("Pro-haloacetate Nanoparticles for Efficient Cancer Therapy via Pyruvate Dehydrogenase Kinase Modulation"). | |
| "With further development, this approach could offer new hope for cancer patients with much desired improvements in their treatment regimen with affordable treatment options," notes Pan. | |
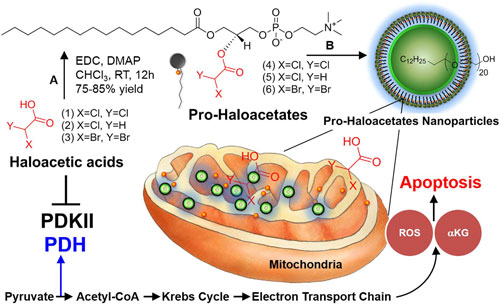 |
|
| Repairing cancer cell suicide mechanism (programmed cell death). A modulation of PDK by nano-enabled delivery of haloacetates. (A) 1-Ethyl-3-(3-dimethylaminopropyl) (© Nature Publishing Group) (click on image to enlarge) | |
| In this work, as part of the quest by nanotechnology researchers to cure cancer, the researchers have demonstrated in a laboratory setting that therapeutic molecules can specifically be delivered to cancer cells in mice by selectively modulating the mitochondrial metabolic function. | |
| The target is a 'gate keeper' enzyme in mitochondria known as pyruvate dehydrogenase kinase (PDK). PDK is activated in a wide range of cancers, which results in the selective inhibition of pyruvate dehydrogenase to suppress mitochondrial apoptosis in cancer cells. | |
| Dichloroacetic acid (DCA) has been used in the past for cancer treatment with conflicting literature reports demonstrating its success. This agent is a common by-product of chlorination of water. | |
| "Since DCA is not a natural agent it is easily absorbed by the body and can even penetrate the blood brain barrier. Due to this rather easy access to the brain, the molecule can pose severe neurological and other toxicity effects," explains Pan. "Our unique approach developed a 'camouflaged' form of DCA, which is not activated until it reaches the intended target; i.e. the cancer cell." | |
| "Furthermore" he adds, "the agent forms tiny nanoparticulates which are selectively taken in by the tumors. We further tested similar compounds to show that not only DCA but other similar halogenated molecules can be used with comparable efficacy." | |
| The team now is in the process of conducting safety studies with the agent. Once the safety is established, the first human studies can be initiated. | |
| Given the fact that these agents are derived from widely available chemicals, with a long history of human use (nearly 40 years), the scientists envision a rapid translation to eventual human use. | |
| These therapeutic agents will be administered in a 'protected' (prodrug) form, which precludes the release of the drug during systemic circulation. By virtue of the tiny size of the nanoparticles derived from these prodrugs, they will be specifically taken up by the 'leaky' tumor vasculature. | |
| The camouflaged form of the drug then becomes activated once it enters the cancer cell. The approach permits use of lower drug dosages, reducing overall toxicity while increasing overall efficacy. | |
| "Lack of selectivity is a key issue in developing drugs for oncology applications," Pan points out. "An average drug discovery process from identification to regulatory approval could easily take more than ten years. It is therefore of utmost interest to the cancer research community to propose potential therapeutic approaches that simultaneously tackle selectivity issues and in parallel quickly translate into an affordable, widely available drug technology." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
