Showing Spotlights 177 - 184 of 203 in category All (newest first):
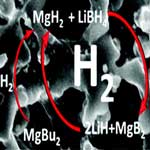 The main obstacle to building a hydrogen economy is the lack of efficient hydrogen storage. The research conducted in the hydrogen storage scientific community is aimed towards mobile applications. Hydrogen is a gas at ambient conditions and takes up a lot of space. For stationary storage facilities, for which available space is not an issue, hydrogen gas can be kept in large tanks at moderate pressures using already known technology. However, in order to utilize hydrogen for mobile applications i.e. to produce and be able to sell hydrogen fueled cars on a large scale, it must be stored in a compact, safe, cheap and efficient way. A European research team has now reported on a new concept for hydrogen storage using nanoconfined reversible chemical reactions. They demonstrate that nanoconfined hydride has a significant hydrogen storage potential.
The main obstacle to building a hydrogen economy is the lack of efficient hydrogen storage. The research conducted in the hydrogen storage scientific community is aimed towards mobile applications. Hydrogen is a gas at ambient conditions and takes up a lot of space. For stationary storage facilities, for which available space is not an issue, hydrogen gas can be kept in large tanks at moderate pressures using already known technology. However, in order to utilize hydrogen for mobile applications i.e. to produce and be able to sell hydrogen fueled cars on a large scale, it must be stored in a compact, safe, cheap and efficient way. A European research team has now reported on a new concept for hydrogen storage using nanoconfined reversible chemical reactions. They demonstrate that nanoconfined hydride has a significant hydrogen storage potential.
Jun 24th, 2010
 One of the greatest current environmental concerns both for the near term as well as for the future is global warming caused by man-made carbon emissions and its well-recognized impact on climate change. The various strategies which can be adopted to combat global warming are classified under the following three categories: 1) Reducing energy consumption by employing more efficient technologies that minimize use of fossil fuels; 2) Adopting technologies that utilize renewable energy and energy storage technologies; 3) Addressing carbon management issues that involve separation, capture, sequestration and conversion to useful products. The present article will specifically address the first two topics.
One of the greatest current environmental concerns both for the near term as well as for the future is global warming caused by man-made carbon emissions and its well-recognized impact on climate change. The various strategies which can be adopted to combat global warming are classified under the following three categories: 1) Reducing energy consumption by employing more efficient technologies that minimize use of fossil fuels; 2) Adopting technologies that utilize renewable energy and energy storage technologies; 3) Addressing carbon management issues that involve separation, capture, sequestration and conversion to useful products. The present article will specifically address the first two topics.
May 4th, 2010
 An intriguing novel approach to extract the energy from the photosynthetic conversion process has been demonstrated by researchers at Stanford and Yonsei Universities. They have inserted ultrasharp gold nanoelectrodes into living algae cells and extracted electrons, thereby harnessing an - albeit very tiny - electrical current. This is electricity production that doesn't release carbon into the atmosphere. The results demonstrate the feasibility of collecting high-energy electrons in steps of the photosynthetic electron transport chain and prior to the downstream processes associated with energy loss. In addition, the system allows direct monitoring of specific charge transfer reactions in live cells, leading to broad applications for investigating developmental processes and the responses of cells and organelles to light and chemical stimuli.
An intriguing novel approach to extract the energy from the photosynthetic conversion process has been demonstrated by researchers at Stanford and Yonsei Universities. They have inserted ultrasharp gold nanoelectrodes into living algae cells and extracted electrons, thereby harnessing an - albeit very tiny - electrical current. This is electricity production that doesn't release carbon into the atmosphere. The results demonstrate the feasibility of collecting high-energy electrons in steps of the photosynthetic electron transport chain and prior to the downstream processes associated with energy loss. In addition, the system allows direct monitoring of specific charge transfer reactions in live cells, leading to broad applications for investigating developmental processes and the responses of cells and organelles to light and chemical stimuli.
May 3rd, 2010
 As it is becoming clearer that one of the critical issues for developing functional nanomachines is the generation of energy required to power them, research into developing nanoscale energy sources has been picking up substantially. The energy to be fed into a nanogenerator is likely to be mechanical energy that is converted into electric energy that then will be used to power nanodevices without using a battery. With the emergence of nanotechnology and the use of nanomaterials, the field of piezoelectrics and nanopiezotronics has experienced a lot of new and interesting research efforts. Researchers in Korea have now demonstrated the first use of chemical vapor deposition-grown large-scale graphene sheets as transparent electrodes for fully transparent and flexible nanogenerators.
As it is becoming clearer that one of the critical issues for developing functional nanomachines is the generation of energy required to power them, research into developing nanoscale energy sources has been picking up substantially. The energy to be fed into a nanogenerator is likely to be mechanical energy that is converted into electric energy that then will be used to power nanodevices without using a battery. With the emergence of nanotechnology and the use of nanomaterials, the field of piezoelectrics and nanopiezotronics has experienced a lot of new and interesting research efforts. Researchers in Korea have now demonstrated the first use of chemical vapor deposition-grown large-scale graphene sheets as transparent electrodes for fully transparent and flexible nanogenerators.
Apr 19th, 2010
 Bioethanol, unlike petroleum, is a form of renewable energy that can be produced from common agricultural feedstocks such as sugar cane or corn. Ethanol is already widely used in siome countries, mainly as biofuel additive for gasoline. The tremendous hype about bioethanol in the past few years has now been followed by a debate about how useful bioethanol actually can replace gasoline. Concerns about its production and use relate to the large amount of arable land required for crops, as well as the energy and pollution balance of the whole cycle of ethanol production. Recent developments with cellulosic ethanol production and commercialization may allay some of these concerns. It offers the promise that any plant material, from grass to wood, and not just edible crops, could be used in the production of ethanol fuels. Consequently, cellulosic ethanol could allow ethanol fuels to play a much bigger role in the future than previously thought. With regard to cellulosic production and commercialization, bioethanol production from woody biomass by enzymatic hydrolysis of cellulosic components and fermentation has attracted much attention.
Bioethanol, unlike petroleum, is a form of renewable energy that can be produced from common agricultural feedstocks such as sugar cane or corn. Ethanol is already widely used in siome countries, mainly as biofuel additive for gasoline. The tremendous hype about bioethanol in the past few years has now been followed by a debate about how useful bioethanol actually can replace gasoline. Concerns about its production and use relate to the large amount of arable land required for crops, as well as the energy and pollution balance of the whole cycle of ethanol production. Recent developments with cellulosic ethanol production and commercialization may allay some of these concerns. It offers the promise that any plant material, from grass to wood, and not just edible crops, could be used in the production of ethanol fuels. Consequently, cellulosic ethanol could allow ethanol fuels to play a much bigger role in the future than previously thought. With regard to cellulosic production and commercialization, bioethanol production from woody biomass by enzymatic hydrolysis of cellulosic components and fermentation has attracted much attention.
Mar 25th, 2010
 Materials that can produce electricity are at the core of piezoelectric research and the vision of self-powering machines and devices. With the emergence of nanotechnology and the use of nanomaterials, the field of piezoelectrics and nanopiezotronics has experienced a lot of new and interesting research efforts. A recent study, for instance, has demonstrated that the small vibrational energy waste generated in the environment from noise, wind power, running water, or water wave action can be scavenged or harvested as a driving force for direct water splitting. The researchers propose a new piezoelectrochemical mechanism for the direct conversion of mechanical energy to chemical energy and subsequently the splitting of water into hydrogen and oxygen.
Materials that can produce electricity are at the core of piezoelectric research and the vision of self-powering machines and devices. With the emergence of nanotechnology and the use of nanomaterials, the field of piezoelectrics and nanopiezotronics has experienced a lot of new and interesting research efforts. A recent study, for instance, has demonstrated that the small vibrational energy waste generated in the environment from noise, wind power, running water, or water wave action can be scavenged or harvested as a driving force for direct water splitting. The researchers propose a new piezoelectrochemical mechanism for the direct conversion of mechanical energy to chemical energy and subsequently the splitting of water into hydrogen and oxygen.
Mar 19th, 2010
 Artificial photosynthesis can offer a clean and portable source of energy supply as durable as the sunlight. Using sunlight to split water molecules and form hydrogen fuel is one of the most promising tactics for kicking our carbon habit. Of the possible methods, nature provides the blueprint for converting solar energy in the form of chemical fuels. A natural leaf is a synergy of the elaborated structures and functional components to produce a highly complex machinery for photosynthesis in which light harvesting, photoinduced charge separation, and catalysis modules combined to capture solar energy and split water into oxygen and hydrogen efficiently. Chinese researchers have now demonstrated the design of an efficient, cost-effective artificial system to mimic photosynthesis by copying the elaborate architectures of green leaves, replacing the natural photosynthetic pigments with man-made catalysts and thereby realizing water splitting- a major advance in energy conversion.
Artificial photosynthesis can offer a clean and portable source of energy supply as durable as the sunlight. Using sunlight to split water molecules and form hydrogen fuel is one of the most promising tactics for kicking our carbon habit. Of the possible methods, nature provides the blueprint for converting solar energy in the form of chemical fuels. A natural leaf is a synergy of the elaborated structures and functional components to produce a highly complex machinery for photosynthesis in which light harvesting, photoinduced charge separation, and catalysis modules combined to capture solar energy and split water into oxygen and hydrogen efficiently. Chinese researchers have now demonstrated the design of an efficient, cost-effective artificial system to mimic photosynthesis by copying the elaborate architectures of green leaves, replacing the natural photosynthetic pigments with man-made catalysts and thereby realizing water splitting- a major advance in energy conversion.
Mar 18th, 2010
 Nanotechnology catalytical techniques are having a profound impact on clean energy research and development, ranging from hydrogen and liquid fuel production to clean combustion technologies. In this area, catalyst stability is paramount for technical application, and remains a major challenge, even for many conventional catalysts. Thermal stability in particular is a challenge across many currently discussed technical applications and an obstacle for many nanocatalyst-enabled devices, from sensors to fuel production. In particular fuel processing technologies (hydrogen and/or liquid fuel production from fossil and renewable resources, clean combustion) typically proceed at particularly severe conditions (high temperatures, high through-put, contaminated fuel streams, etc) and hence require particular attention to catalyst stabilization, but even many processes at much lower temperature conditions, such as fuel cells, are still looking for catalysts with improved stability.
Nanotechnology catalytical techniques are having a profound impact on clean energy research and development, ranging from hydrogen and liquid fuel production to clean combustion technologies. In this area, catalyst stability is paramount for technical application, and remains a major challenge, even for many conventional catalysts. Thermal stability in particular is a challenge across many currently discussed technical applications and an obstacle for many nanocatalyst-enabled devices, from sensors to fuel production. In particular fuel processing technologies (hydrogen and/or liquid fuel production from fossil and renewable resources, clean combustion) typically proceed at particularly severe conditions (high temperatures, high through-put, contaminated fuel streams, etc) and hence require particular attention to catalyst stabilization, but even many processes at much lower temperature conditions, such as fuel cells, are still looking for catalysts with improved stability.
Dec 4th, 2009
 The main obstacle to building a hydrogen economy is the lack of efficient hydrogen storage. The research conducted in the hydrogen storage scientific community is aimed towards mobile applications. Hydrogen is a gas at ambient conditions and takes up a lot of space. For stationary storage facilities, for which available space is not an issue, hydrogen gas can be kept in large tanks at moderate pressures using already known technology. However, in order to utilize hydrogen for mobile applications i.e. to produce and be able to sell hydrogen fueled cars on a large scale, it must be stored in a compact, safe, cheap and efficient way. A European research team has now reported on a new concept for hydrogen storage using nanoconfined reversible chemical reactions. They demonstrate that nanoconfined hydride has a significant hydrogen storage potential.
The main obstacle to building a hydrogen economy is the lack of efficient hydrogen storage. The research conducted in the hydrogen storage scientific community is aimed towards mobile applications. Hydrogen is a gas at ambient conditions and takes up a lot of space. For stationary storage facilities, for which available space is not an issue, hydrogen gas can be kept in large tanks at moderate pressures using already known technology. However, in order to utilize hydrogen for mobile applications i.e. to produce and be able to sell hydrogen fueled cars on a large scale, it must be stored in a compact, safe, cheap and efficient way. A European research team has now reported on a new concept for hydrogen storage using nanoconfined reversible chemical reactions. They demonstrate that nanoconfined hydride has a significant hydrogen storage potential.
 Subscribe to our Nanotechnology Spotlight feed
Subscribe to our Nanotechnology Spotlight feed





