| Posted: Apr 21, 2009 | |
Pinpointing catalytic reaction sites on carbon nanotube walls |
|
| (Nanowerk Spotlight) Carbon nanotubes have been recognized as promising materials for catalysis, either as catalysts themselves, as catalyst additives or as catalyst supports. Researchers are particularly excited by the promise of single-walled carbon nanotubes (SWCNTs) as electrocatalysts or electron harvesting materials for electron-to-fuel conversion. | |
| Nanotubes act as catalysts when an electric current is passed through them. This enables them to donate electrons to molecules that come in contact with the reaction sites. In trying to explain carbon nanotubes' role as catalyst, scientist have long proposed that the reactive sites for catalysis can be discrete, specific sites rather then the entire sidewall of the nanotubes. But observing them directly has been challenging. | |
| A team at Cornell University has now filled in an important blank by pinpointing unique sites where the reactions take place on SWCNTs. The scientists showed that the reactions do not occur all along the tubes, but at the ends of the tubes or at defects along the tubes. | |
| "Employing single-molecule fluorescence microscopy of SWCNT electrocatalysis at single-reaction resolution allowed us the multiplexed observation of electrocatalysis by many SWCNTs in real time," Peng Chen tells Nanowerk. "We found that the electrocatalytic reactions occur at discrete, nanometer-dimension reactive sites on the SWCNTs, where the enhanced mass transport allows measurements of steady-state electrochemical electron-transfer kinetics." | |
 |
|
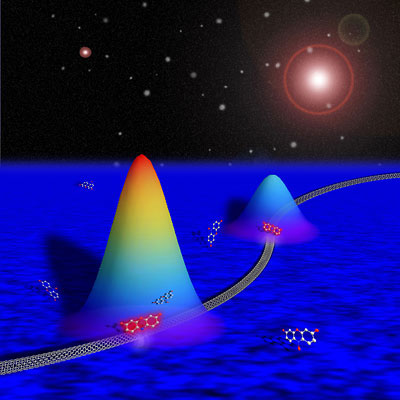
| Artist's depiction of reactions occurring along a single-walled carbon nanotube converting a nonfluorescent molecule into a fluorescent one. Each "mountain" schematically depicts the spatial profile of the fluorescence intensity of a single product molecule at a reactive site. Determining the peak position of the mountain allows researchers to pinpoint the reaction site to within as little as 20 nanometers. (Image: Hao Shen, Cornell University) | |
| To monitor the electrocatalysis by SWCNTs at the single-molecule level, Chen's group used an electrochemical flow cell and a wide-field total internal reflection fluorescence microscope. They trapped an array of nanotubes between transparent conductors in a solution of resazurin and made a 'movie' with an exposure every 100 milliseconds over tens of minutes after applying a voltage to start the catalytic reaction. A scattering of bright dots in each frame shows that the reactions are not happening all along the tubes. | |
| The team then plotted the rise and fall of brightness across each fuzzy dot to pinpoint the center. Finally, they superimposed the centers from all the frames of the movie and repeated the process to refine the locations to within 20 nanometers or less. | |
| In their work, just published online in Nano Letters ("Single-Molecule Electrocatalysis by Single-Walled Carbon Nanotubes"), Chen, an assistant professor of chemistry and chemical biology at Cornell, and his group report several scientific achievements: | |
| 1) The ability to monitor the electrocatalytic reaction by single-walled carbon nanotubes at single-reactive site, single-reaction resolution in real time under ambient conditions. This was never achieved previously. | |
| 2) The demonstration that these electrocatalytic reaction occur at discrete sites, possibly defect sites or nanotube ends, rather than on the normal sidewall of the nanotubes. | |
| 3) With super-resolution optical imaging technique, enabled by their single-molecule detection, the team was able to resolve the physical dimension of the reaction sites down to 20 nm, an order of magnitude better than the normal diffraction-limited optical resolution. | |
| 4) The ability to quantify the electrocatalytic kinetics and the associated the electron transfer process at each reactive site. This enabled Chen's team to quantify the reactivity of each reactive site and obtain the reactivity distribution. | |
| "Our approach can be generalized to study many other electrocatalytic materials in order to gain unprecedented knowledge about their fundamental properties," says Chen. "For one thing, in electrocatalysis, electron transfer from the nanotube to an adsorbed molecule is a fundamental process, which we can measure in our experiments. We are now looking into how the electron transfer depends on the applied electric potential, to understand the interfacial electron transfer process." | |
| Having demonstrated that they can now study the electrocatalytic activity at the single nanotube, single reaction level, a next step for Chen's team is to combine these findings with structural and electronic measurements of individual tubes to identify what the chemical nature of the reactive sites is and how the structure and electronic properties of nanotubes affect their reactivity. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
