| Posted: Oct 16, 2009 | |
Early-stage detection of cancer cells without biopsy |
|
| (Nanowerk Spotlight) Detection at the earliest stage provides the greatest chance of survival for cancer patients. Cancer has a logarithmic growth rate and doctors typically don't see 80% of the life of a tumor. Detection can be done using a number of techniques including standard immunoassays and biopsies. Nanotechnology offers new detection approaches such as targeted contrast agents, nanoscale cantilevers coated with antibodies against tumor markers, and magnetic nanoparticles coated with DNA labeling. But the problem is daunting because there are over 50 common types of cancer and in practice it is difficult to ask people to come to the clinic on a regular basis for cancer screening. | |
| "To date, the methods for detection of cancer cells are mostly based on traditional techniques used in biology, such as visual identification of malignant changes, cell-growth analysis, specific ligand-receptor labeling, or genetic tests," Igor Sokolov, a professor of physics and director of the NanoBioLaboratory (NABLAB) at Clarkson University, tells Nanowerk. "Despite being well developed, these methods are either insufficiently accurate or require a lengthy complicated analysis, which is impractical for clinical use. There is a hope that physical sciences can show a way for alternative methods of detection of cancer cells, which will be more precise and simpler than the traditional methods. The method that we have reported recently falls in category of those alternative methods." | |
| Sokolov and his team have proposed a new method for the detection of cancer cells based on measurement of the physical adhesion of silica beads to malignant versus normal cells cultured from human cervix. The difference between healthy and tumor cells was first studied by atomic force microscope (AFM). Then the team expanded their method to use fluorescent silica particles, and the corresponding difference in the number of adherent particles was detected by a simple fluorometry method. | |
| "Our finding is based on our research results recently published in Nature Nanotechnology ("Atomic force microscopy detects differences in the surface brush of normal and cancerous cells"), where we reported on observation of the difference in surface physical properties of cancerous and normal human epithelial cervical cells," explains Sokolov. "Specifically, we reported quantitative differences in the brush layer on the cell surface. These brush layers, which consist mostly of microvilli, microridges, and cilia are important for interacting with the environment. We found that normal cells have brushes with one length while cancerous cells displayed long and short brushes with significantly different densities." | |
 |
|
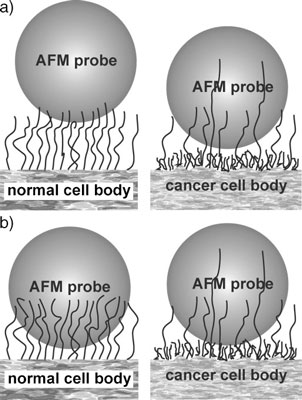
| Schematic diagram of the interaction between a silica particle and brushing cell surface. The areas of contact between the cellular membrane and a silica particle are different for cancerous and normal cells. a) The initial contact ofasilicaAFMprobeand cell surface. b) After a period of contact, the silica AFM probe becomes enveloped by the long brush of normal cells, but it can still be repelled by the fairly dense and short second (inner) brush of cancer cells. (Reprinted with permission from Wiley-VCH Verlag) | |
| These findings led to the present work where the team expected the difference in the brush to lead to the differences in the adhesion of various particles to such cells. | |
| Reporting their work in a recent issue of Small ("Towards Nonspecific Detection of Malignant Cervical Cells with Fluorescent Silica Beads"), Sokolov and his team demonstrated that the adhesion depends on the duration of the contact between the silica particles and cell surface. Due to the difference in adhesion, a different number of silica particles adhere to malignant versus normal cells. | |
| As a result, the adhesion of the particles to cells is statistically different for cancerous and normal cells – a result that can be used in developing a fairly uncomplicated cancer detection method that does not require a biopsy. | |
| "We have also used ultrabright fluorescent silica beads to make the detection of silica particles easy" says Sokolov. "Here, instead of having to count the number of adherent particles, one can measure the total amount of fluorescent light coming from a particular area. The measurement of fluorescent light is straightforward because the particles emit a strong fluorescence signal. This can be achieved with a fairly simple instrumental setup and does not require the high-sensitivity spectrometer typically used for measuring fluorescence." | |
| The method developed by the Clarkson team is nontoxic due to the encapsulation of the fluorescent dye within the silica particles, and it does not use any modification of the silica surface for tagging. Other advantages are that this method has a relatively high specificity, is simple, comparatively inexpensive, and straightforward in interpretation. | |
| Sokolov cautions that, although the method shows reproducible statistical differences between cancerous and normal cells, more statistical data is needed to determine the actual efficiency of the method. | |
| "The problem is in the variability of human subjects" he says. "We did find unambiguous difference of three healthy individuals and three cancer patients. More statistics has to be collected before we can speak about clinical applications." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
