| Posted: Sep 22, 2010 | |
A magnetic leaf |
|
| (Nanowerk Spotlight) The exquisite microstructures created by nature may hold the answer to a complex synthetic challenge. Recently, our group reported the use of fig leaves to create intricate networks of the magnetic material iron carbide ("Biotemplating of Metal Carbide Microstructures: The Magnetic Leaf"). This simple technique could be used to make a new generation of carbide materials. | |
| Metal carbides are a fascinating class of materials with properties as diverse as hardness, conductivity or catalytic activity. They already have many common uses, such as tungsten carbide for wear-resistant drill bits. However, future applications of these materials demand structural control on the micro and nano-scale. For example, medical imaging techniques require uniform nanoparticles of iron carbide whereas catalytic or electrochemical applications would need a porous, high surface-area monolith. | |
| There are many examples of nanoparticles, nanowires or porous structures of metal oxides but few corresponding reports for carbides. This is partly because carbides require very high temperatures for synthesis, which makes controlling the crystal growth a challenge. | |
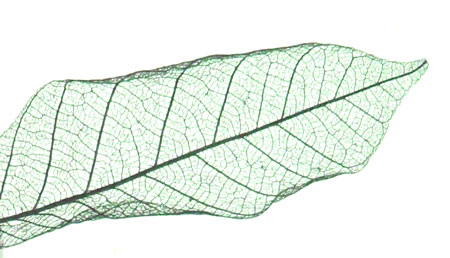
| In this research, we demonstrated a simple procedure to create a complex, hierarchical microstructure of iron carbide using a biological template. By soaking a leaf skeleton in an iron acetate solution, followed by heating under nitrogen, an intact 'magnetic leaf' could be made. | |
 |
|
| A magnetic leaf: UA simple chemical process converts a leaf skeleton into iron carbide, which is magnetic and conducts electricity. (Image: Max Planck Institute of Colloids and Interfaces) | |
| The basis for our technique is extremely simple. The heating process initially breaks down the biological polymers in the leaf and converts the iron acetate to iron oxide. This iron oxide coating then reacts with the carbon-rich leaf template, forming an even layer of iron carbide in an exact replica of the original leaf. Remarkably, the original biological structures such as the xylem could be seen clearly using a microscope. | |
| Since iron carbide is conductive as well as magnetic, we also demonstrated the use of the leaf as an electrode in a simple water splitting process. The base of the leaf was soldered to a copper wire and the tip submerged in the aqueous electrolyte of an electrochemical cell. Applying a voltage across the cell resulted in the evolution of bubbles of hydrogen and oxygen. The conductivity of the leaf electrode showed that the iron carbide had formed an even layer over the entire structure. | |
| While the magnetic leaf may itself find some applications, the real importance of this work is as a general route to complex metal carbide microstructures. Nature offers many fascinating carbon-rich structures and it is also possible to generate fibres, networks or porous sponges of synthetic polymers. By selecting a template with specific structural characteristics, it should be possible to transfer these characteristics directly into a metal carbide product. | |
| For example, a highly porous sponge template could be used to generate a high surface-area carbide catalyst. These are particularly exciting since they may offer a cheap and sustainable alternative to noble metals such as platinum in fuel cell electrodes. | |
| By Dr. Zoë Schnepp, Max Planck Institute of Colloids and Interfaces, Department of Colloid Chemistry | |
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
