| Posted: Sep 03, 2012 | |
Unique porous carbon spheres made by ultrasonic spray pyrolysis |
|
| (Nanowerk Spotlight) Ultrasonic spray pyrolysis (USP) has been widely used in industry for spherical solid powder production, particularly of metal oxides. For some applications, though, porous particles are more desirable than dense ones. Back in 2005, Ken Suslick's research group at the University of Illinois developed a technique to synthesize porous micro- and nanoparticles via USP. | |
| This method has since been expanded to prepare porous carbon microspheres (see for instance: "New method for making tiny catalysts holds promise for air quality"). Last year, Suslick and his team developed a continuous, one-step and template-free aerosol approach using USP to prepare porous carbon spheres. The high surface area and unique porous structures suggest that porous carbon spheres can be useful for electrode materials, adsorbents, and catalyst supports. The University of Illinois team already demonstrated the use of carbon microspheres as supercapacitors. | |
| In their latest work, the group has now has now expanded the aerosol synthesis of porous carbon materials by the use of energetic carbon precursors. Some of the resulting porous carbon spheres exhibit unique and unprecedented morphologies. | |
| "By varying the compositions of carbon precursors in ultrasonic spray pyrolysis, we are able to make a number of completely new porous carbon structures and morphologies," Suslick explains to Nanowerk. "Compared to other procedures for porous carbon synthesis, this approach is a one-step process without exotemplates and it doesn’t require a series of tedious steps – e.g., preparation of porous templates first, infiltration of carbon precursors, carbonization, and template removal." | |
| As they reported in the August 24, 2012 online edition of Advanced Materials ("Porous Carbon Spheres from Energetic Carbon Precursors using Ultrasonic Spray Pyrolysis"), the researchers used propiolate salts containing C≡C bonds that are fuel-rich, high-energy precursors, and their thermal decomposition pathways are dramatically different from the usual activated carbon precursors. | |
 |
|
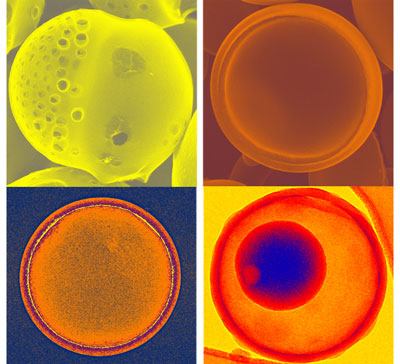
| Porous carbon microspheres prepared from energetic carbon precursors using ultrasonic spray pyrolysis. Small differences in the precursor mixtures of alkali propiolates can lead to the dramatically different carbon structures and morphologies. (Image: Hangxun Xu and Kenneth S. Suslick, University of Illinois) | |
| "Their vigorous off-gassing behaviors during pyrolysis are especially interesting to generate porous carbon structures," says Suslick. "In this recent work, we discovered a series of carbon structures and morphologies such as Janus, jellyfish-like, and hemispherical nested double-bowl carbon microspheres that have never been reported before. Some carbon morphologies that we reported are new to the carbon community and have never been produced using other methods. " | |
| He notes that the morphologies of the porous carbon materials resulting from the thermolysis of the alkali propiolates are dependent on choice of propiolate cations. This phenomenon is caused by the differences in the thermal decomposition of the precursors. | |
| According to the team, the most challenging part of this work has been to elucidate the mechanism for the formation of unique carbon spheres when different alkali propiolates are mixed together as carbon precursors. | |
| "Very little changes in the precursor compositions can lead to the generation of dramatically different carbon microstructures and morphologies," says Suslick. "It is difficult to predict what the next carbon microspheres will look like if we mix different or multiple precursors together or use different weight ratios. The use of thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) to understand the thermal decomposition behaviors of individual alkali propiolates or mixed salts has been partially successful in providing reasonable explanations for observed morphologies, but our understanding remains rather incomplete." | |
| In this work, Suslick's group focused on using CH≡CCOOLi, CH≡CCOONa, CH≡CCO2K and their mixtures as carbon precursors. However, there are many other salts and related precursors – for example yielding C3, rather than C2 intermediates, and their mixtures as carbon precursors. | |
| "Furthermore, we would also like to incorporate some functional nanomaterials into our porous carbon microspheres," says Suslick. "For example, we can make tin nanoparticles imbedded porous carbon microspheres as anode materials for lithium-ion batteries, making them in a single-step using both tin and carbon precursors. As mentioned earlier, the challenge is to systematically understand why the carbon microspheres form the particular structures or morphologies. " | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
