| Posted: Jul 24, 2017 | |
How to detect biological contamination of nanoparticles |
|
| (Nanowerk Spotlight) Given the growing impact of nanotechnology on health care, pharmacy, and medicine, there is an increasing and urgent need for the development of reliable methods to detect the biological contamination of nanoparticles. | |
| Engineered nanomaterials such as carbon nanotubes, fullerenes, graphene nanosheets, quantum dots, dielectric and metal nanoparticles hold great promise for a variety of biomedical applications. The most well-known are diagnostic and therapeutic applications. | |
| For example, semiconductor quantum dots and metal (silver and gold) nanoparticles can both detect and destroy pathogenic microbes. Similarly, nanohybrids made of metals and semiconductors can also be used in fighting tumors (the concept of photodynamic therapy). Another very promising way to kill tumor cells is hyperthermia treatment mediated by magnetic nanoparticles. | |
| In addition, magnetic nanomaterials are indispensable to improve the sensitivity and contrast of magnetic resonance imaging. Nanomaterials can also be used as nano-vehicles for targeted drug delivery and in tissue engineering. | |
| When it comes to diagnostic and therapeutic applications of nanomaterials, the assessment of their cytotoxicity is a standard and required procedure. Typically, engineered nanomaterials are not sterile and can be contaminated with biological species such as endotoxins. | |
| Endotoxins (or bacterial lipopolysaccharides) are large and heat-stable molecules which are the major component of the Gram-negative bacteria cell wall. An exposure to endotoxins can cause fever, septic shock, and even death. | |
| "It should be noted that the contamination of engineered nanomaterials with endotoxin can easily happen at any stage of their production and handling," Yuriy Garbovskiy, PhD, a researcher at the UCCS BioFrontiers Center & Department of Physics, University of Colorado, tells Nanowerk. "This biological contamination of nanoparticles, if not detected, can alter the results of the cytotoxicity test routinely used to characterize these nanomaterials. As a result, the cytotoxicity of 100% pure and bio-contaminated nanoparticles can differ very significantly." | |
| That is why existing protocols and good laboratory practice require testing engineered nanomaterials for sterility and pyrogenicity – the capacity to cause fever – to assess their possible contamination with endotoxins. | |
| Available commercial chromogenic assays for the detection and quantification of bio-contaminants such as endotoxin can interfere with nanoparticles thus leading to unreliable data. Consequently, there is a strong need for the development of new ways to assess the level of the biological contamination of engineered nanomaterials. | |
| Garbovskiy recently published a paper in Analytical Chemistry ("Biological Contamination of Nanoparticles and Its Manifestation in Optical Absorbance Measurements") that offers a solution to the aforementioned problems of the nanoparticle interference and correct quantification of bio-contaminants such as endotoxins. His proposed method is based on optical absorbance measurements. | |
 |
|
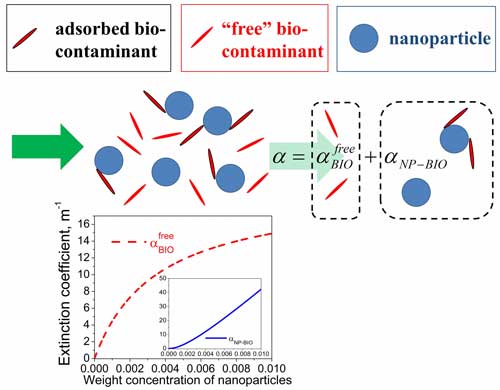
| Schematic illustration of the proposed approach to deduce the level of the biological contamination of nanoparticles. (Image: Dr. Yuriy Garbovskiy, University of Colorado, Colorado Springs). | |
| "The basic idea of this paper is very simple," says Garbovskiy. "Once contaminated nanoparticles are dispersed in the contaminant-free dispersion medium, some fraction of the bio-contaminants adhering to the nanoparticle surface becomes detached from the surface and freely dispersed in the medium. As a result, the measured optical absorbance of the system under study (contaminated nanoparticles dispersed in the dispersion medium) can be written as a sum of two components". | |
| "The first component accounts for the absorbance of light by freely dispersed bio-contaminants (dashed curve in the Figure above)," he continues. "This component can be used to assess the biological contamination of nanoparticles. The second component originates from the combined absorbance of light by nanoparticles and the bio-contaminants attached to them (solid curve)." | |
| It should be noted that these two components are physically measurable quantities as illustrated by the Figure above. | |
| The paper provides an analysis of how the level of the biological contamination of nanoparticles can be assessed by analyzing the dependence of the optical absorbance of contaminated nanoparticles on the concentration of nanoparticles and on the constant characterizing the binding of bio-contaminants to the surface of nanoparticles. | |
| Garbovskiy's proposed model should be of great interest to researchers trying to apply optical techniques for the detection and quantification of the biological contamination of nanomaterials. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
