| Apr 26, 2019 | |
Intercalation energy tuning improves performance of multivalent ion batteries |
|
| (Nanowerk Spotlight) Aqueous rechargeable batteries based on earth abundant materials are promising alternatives to lithium-ion batteries, which are plagued by safety and environmental concerns. These batteries utilize water-based electrolytes, which are safe, low-cost, and environment-friendly, but also possess a much higher ionic conductivity than that of the organic electrolytes. | |
| In particular, aqueous zinc-ion batteries have attracted increasing attention among researchers because of the distinctive merits of zinc (Zn) metal anodes, such as low cost and high global production, high energy density, and relatively low electrode potential. Unfortunately, this type of battery suffers from the lack of suitable cathode materials because of the sluggish intercalation kinetics associated with the large size of hydrated zinc ions. | |
| In a paper in Nano Letters ("Aqueous Zinc-Ion Storage in MoS2 by Tuning the Intercalation Energy"), researchers at King Abdullah University of Science & Technology (KAUST) report an effective and general strategy to transform inactive intercalation hosts into efficient Zn2+ storage materials through intercalation energy tuning. | |
| "The most exciting findings of our work are that we boosted the Zn2+ storage capacity of barely active MoS2 by 10 times through simple interlayer spacing and hydrophilicity engineering," Husam N. Alshareef, a Professor of Materials Science & Engineering, tells Nanowerk. "Our work suggests a general strategy to greatly boost the charge storage capacity of intercalation hosts. The findings will facilitate the particle application of the large family of layered materials in energy storage devices, not only the challenging multivalent batteries (e.g. Zn2+, Mg2+, Al3+), but also the more established Li+ and Na+ batteries." | |
| Using one of the most extensively studied Li/Na host materials – the layered chalcogenide MoS2 – as a model compound, the team shows both computationally and experimentally that the interlayer spacing and hydrophilicity tuning, which are achieved by oxygen incorporation, effectively boost the Zn2+ diffusion kinetics by 3 orders of magnitude. | |
| The incorporation of oxygen into MoS2 improves hydrophilicity, which makes the water intercalation into intrinsically hydrophobic MoS2 possible to proceed at low reaction temperatures. As a result, we achieved an enlarged interlayer spacing of MoS2, from 0.62 to 0.95 nm, and a decreased water contact angle from 127° to 110ˆ°,” said Dr. Hanfeng Liang, lead author of the study. | |
| This further greatly increases the interlayer spacing of MoS2 from 0.62 to 0.95 nm and decreases the water contact angle from 127° to 110°. | |
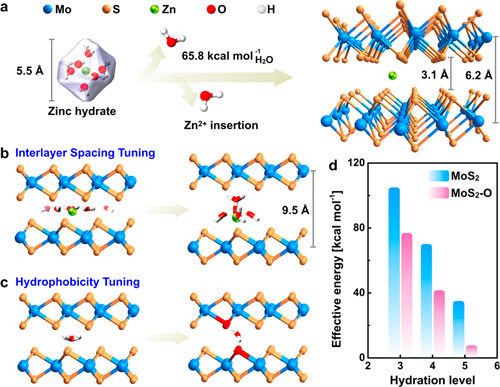 |
|
| Schematic illustration of strategies developed in this work to enhance Zn2+ diffusion kinetics. (a) In pristine MoS2, the interlayer spacing is limited, and thus, the intercalation of Zn hydrate is unlikely to proceed due to the huge energy barrier, resulting in the considerably low Zn2+ storage capacity. (b) Interlayer expanded MoS2 has a significantly lower Zn2+ intercalation energy because of the large scale of preservation of the Zn hydrate structure. (c) Hydrophilicity tuning strengthens the ZnII-H2O-O interaction and therefore promotes Zn2+ diffusion. (d) Theoretically calculated effective energy against the hydration level of Zn2+. (Reprinted with permission by American Chemical Society) (click on image to enlarge) | |
| "Our simulation result suggests that the intercalation energy of Zn2+ in the modified MoS2 significantly reduces from 104.5 to 7.8 kcal mol-1 compared to pristine one," Alshareef points out. "We confirmed experimentally that a 5% oxygen incorporation into MoS2 boosts Zn2+ diffusivity by 3 orders of magnitude, effectively enabling the otherwise barely active MoS2 to achieve a high capacity of 232 mAh g-1 that is 10 times as its pristine form (∼21 mAh g-1)." | |
| He adds that the promoted Zn2+ intercalation in aqueous solution also promises an efficient and safe way to produce the metallic 1T MoS2 (with a yield of 78%), which has found various promising applications but suffers from the safety issue in its preparation by the common Li+ intercalation method. | |
| "Our discoveries will promote the use of MoS2 in aqueous zinc ion batteries or even other multivalent ion batteries," said Dr. Hanfeng Liang, lead author of the study. "The produced metallic 1T MoS2 can readily find many applications such as batteries, electrocatalysis, and sensors." | |
| One potential issue with this design could be that the intercalation energy tuning of MoS2 also enhances its electrocatalytic activity. The modified MoS2 therefore might catalyze some side reactions during battery operation and affect the battery performance. The researchers are confident, though, that this can eventually be solved by electrode surface engineering (e.g. coatings, plasma treatments). | |
| The tam plans to move this research forward in several directions: 1) surface engineering (e.g. coatings) to suppress the electrocatalytic activity of MoS2; 2) fabricating flexible MoS2 based Zn ion batteries; 3) extending the concept to other intercalation hosts for electrochemical energy storage. | |
| "Although aqueous zinc ion batteries present low-cost, safe, and high-energy battery technology and already many exciting results have been achieved, there is still a long way to go to for achieve practical applications," Alshareef concludes. "This requires substantial improvements in many aspects ranging from battery design to cathode/anode/electrolyte and even separator optimization, as well as the fundamental understanding of Zn2+ storage mechanisms." | |
| he lists some of the challenges facing future research in this area: 1) facilitating the Zn2+ intercalation kinetics of cathode materials but at the same suppressing the electrocatalytic activity to achieve a wide operating voltage window; 2) optimizing Zn anodes to achieve long-term operation stability; 3) exploration of low cost electrolytes that can reduce the side reactions; and 4) developing in situ and operando characterization techniques to track the reaction pathways and to probe the intermediates. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
