| Posted: Jul 15, 2008 | |
Photoactive graphene-semiconductor composites |
|
| (Nanowerk Spotlight) The electrical properties of graphene have been the topic of recent interest from various disciplines because this novel carbon material offers exciting opportunities to develop nanocomposites with unusual electronic catalytic properties. Most of these studies involve mechanical peeling of individual graphene sheets – one at a time – from a block of graphite. | |
| The requirement to obtain graphene as individual sheets and to maintain it in the reduced form introduces a certain level of complexity into the process of designing composite systems where, for instance, semiconductor or metal nanoparticle are anchored on graphene sheets. Without some form of intervention, the strong van der Waals interactions between reduced graphene sheets would cause them to collapse and aggregate. | |
| Researchers have now developed a simple photocatalytic method to anchor semiconductor nanoparticles on a single sheet of graphene using a solution-based process. | |
| "Following our recent report ("Decorating Graphene Sheets with Gold Nanoparticles") on the formation of a graphene-gold composite system that is suspendable in an aqueous medium using a chemical reduction method, we have now designed an on-demand reduction strategy to obtain graphene semiconductor composite nanostructures," Dr. Prashant V. Kamat tells Nanowerk. "We successfully carried out UV-induced photocatalytic reduction of graphene oxide and maintained well-separated graphene-semiconductor composite sheets." | |
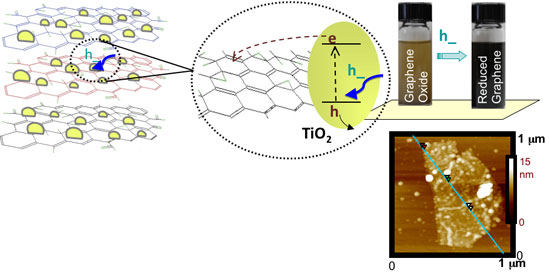 |
|
| Left and center: TiO2-graphene nanocomposite and its response under UV-excitation. Upper right: The change in color of a solution of TiO2 nanoparticles with graphene oxide before and after UV irradiation for 2 hours. Bottom right: AFM (atomic force microscopy) image of single graphene sheet with bound TiO2 nanoparticles. (Image: Dr. Kamat, Notre Dame University) | |
| Kamat, a professor of Chemistry & Biochemistry at Notre Dame University in Indiana, and a senior scientists at the university's Radiation Laboratory, and his team report their latest study, in which they prepared a mixture of graphene oxide and colloidal TiO2 in ethanol and subjected it to steady state UV-irradiation, in the July 3, 2008 online edition of ACS Nano ("TiO2-Graphene Nanocomposites. UV-Assisted Photocatalytic Reduction of Graphene Oxide"). | |
| "Graphene oxide suspended in ethanol undergoes reduction as it accepts electrons from UV-irradiated TiO2 suspensions" explains Kamat. "The reduction is accompanied by changes in the absorption of the graphene oxide, as the color of the suspension shifts from brown to black. The direct interaction between TiO2 particles and graphene sheets hinders the collapse of exfoliated sheets of graphene." | |
| With this technique, on-demand reduction can be triggered by simply turning on the UV light. This reduction method is also relatively mild compared to the conventional approach of using elevated temperatures or chemicals for reducing graphene oxides. | |
| This simple strategy, mainly based on a solution-processable method that is easy to implement for a large scale operation, opens up new ways to anchor photocatalytic nanoparticles on a conducting carbon 'nanomat' (each graphene sheet is a 2-dimensional carbon mat that is only about one nanometer thick). | |
| Carbon supports are often used to disperse catalyst particles – for example, platinum nanoparticles dispersed on carbon particles in fuel cell membrane electrodes. A good dispersion of the catalyst particles is crucial for maximizing the catalyst activity. Deposition of semiconductor or metal nanoparticles on graphene sheets can provide new ways to disperse catalyst materials on a carbon support and carry out the catalytic process efficiently. | |
| Kamat, whose research is supported by the Office of Basic Energy Sciences of the US Department of Energy, points out that improving the efficiency of photoelectrolysis of water remains a challenge. "Semiconductor particles dispersed on graphene not only exhibit improved photocatalytic performance but such composites can also improve the conductivity of graphene sheets or enable development of sensor materials." | |
| Going forward, the aim of Kamat's team is to develop semiconductor-graphene composites for light-energy conversion (solar cells and solar hydrogen production). "The presence of graphene can make a photocatalytic process more efficient by improving the charge separation," he says "and efforts are also underway to utilize metal-graphene composites in fuel cell applications." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com.
