| Posted: Oct 03, 2008 | |
Exploring protein activity on a nanotechnology needle tip |
|
| (Nanowerk Spotlight) Genomics and proteomics, the studies of genes and proteins, provide the underlying basis for many advances in drug development and effective treatments of diseases. These studies heavily rely on unveiling the behavior of a single DNA or protein in an investigative sample. You could compare this challenge to somehow finding, then catching and monitoring a particular fish in a vast ocean. The scientific term for 'catching the fish' is 'immobilization' – a powerful technique for the study of biochemical systems that allows for the continuous observation of dynamic behavior of a chosen target. Immobilization methods anchor the to be observed molecule onto a surface in order to restrict it from escaping the observation volume. | |
| A number of challenges remain in achieving suitable immobilization and isolation of biological molecules without compromising their structure, function, activity, or signal. For instance, surrounding molecules can cause interference which leads to biased signals. Also, the anchored molecules change their natural conformation which affects the accuracy of the collected data. | |
| While for years scientists have tried to improve the way of creating virtual isolation for a molecule, a group of researchers has now developed a new platform which consists of a carbon nanotube nanoneedle for capturing, isolating and measuring the activity of miniscule amounts of proteins. | |
| "The unique features of our new method are (a) the immobilization of a few molecules; (b) subsequent translocation and isolation of them near the tip of a position-actuated nanoneedle; and (c) a fixed, defined, and unhindered molecular position to allow rapid real-time sensing and monitoring" Dr. Anubhav Tripathi tells Nanowerk. | |
| The experiments reported by Tripathi, Assistant Professor of Engineering and of Medical Science and Director of Undergraduate Studies in Biomedical Engineering at Brown University in Providence, Rhode Island, were conducted using an ensemble of proteins immobilized on the nanoneedle tip. | |
| Nanoneedles are ultra thin needle-like devices made out of carbon nanotubes. The sharpness of these nanoneedles can be as small as a couple of nanometers at the tip which is comparable to the size of biological molecules. | |
| "Anchoring of proteins or DNA at such sharp surfaces helps the molecules to maintain their natural form and activity as we have demonstrated in our work" explains Tripathi. "Due to the naturally existent bonds on the carbon nanotubes, the anchoring process is a simple matter of well known biochemistry. The space available for the molecules to sit on the nanoneedle is so small that only very few of them can anchor. After successful anchoring, the nanoneedle can be easily moved out from the liquid sample containing unbound molecules. The nanoneedle can then be placed into a fresh and clean liquid. The data acquisition can the be performed in practically background/noise free manner on very few numbers of isolated molecules." | |
| This work, which shows the promise of high-sensitivity monitoring of enzymatic activity and the preservation of protein viability, was published in Langmuir ("Nanoneedle Method for High-Sensitivity Low-Background Monitoring of Protein Activity"). | |
| "Although not yet achieved in our work, our results represent a significant advance toward reaching the ultimate potential offered by this methods – performing single-enzyme investigations" says Tripathi. | |
| He points out that the nanoneedle is an assembly of many carbon nanotubes and the needle apex consists of multiple opened ends of integrated carbon nanotubes. Although the researchers cannot exclude that some of the proteins attached to defect sites at the nanotubes, they think it is most likely that they were immobilized on and surrounding the tips of the component carbon nanotubes. To reduce the nonspecific adsorption of proteins to the sidewall of the nanotube nanoneedle they coated the side walls with blocking agents. | |
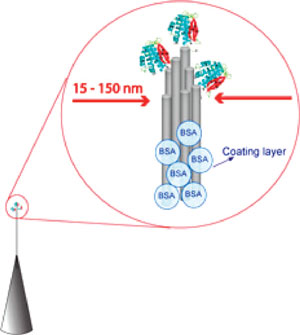
|
|
| Schematic illustration of proteins (not to scale) bound to the nanoneedle tip. It is important to note that the 15 nm tip was likely to be composed of tips of a few single-walled carbon nanotubes of diameter close to 1 nm. Hence, such tips were sharp enough to provide singlepoint contacts for AP molecules (∼5 nm). (Reprinted with permission from American Chemical Society) | |
| "Physically, the number of immobilized proteins in this nanoneedle platform can approach a few proteins with a shorter exposed length at the tip after the coating, or shorter loading time during the immobilization steps, or a smaller voltage applied to the tip, or a lower concentration of the target protein solutions" Tripathi explains. | |
| Although the number of immobilized biotin-AP molecules could be lowered all the way down to the single-molecule level, in this work the number of immobilized molecules was limited by the sensitivity of the fluorometer apparatus and by the finite viable lifetime or stability of the alkaline phosphatase enzyme. | |
| According to the Brown University team, the nanoneedle method introduced in their work offers essentially unhindered access to the proteins, elimination of background signal from the otherwise coexisting molecules, and a fixed, defined molecular location to allow rapid, real-time detection for reaction kinetics or activity studies. | |
| "A faster, easier, cheaper and more accurate way of doing protein and genome studies are some of the future applications of nanoneedles" says Tripathi. "We believe, nanoneedles offer a new and practical path for broadening the horizon of functional protein, DNA and RNA studies." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com.
