| Posted: Sep 28, 2006 | |
Nanoscale prisons lock up molecules |
|
| (Nanowerk Spotlight) Imagine to catch one, or a few, molecules dissolved in water, lock them up in a cage with a diameter of a few hundred nanometers, and keep them locked for a given length of time. Then bring these containers with the "captive" molecules to places within the solution where you want to have them, and release the captured molecules from their captivity on chemical command. Or simply keep the molecules in the cage "prison" locked up, add a few more different molecules to water, and watch their chemical reaction following movement across the container wall in "solitary" confinement within the containers with the molecules already captured. Such dreams of nanotechnologists have come much closer to reality as a result of a discovery made by a team of researchers, lead by Professor Julius Vancso of the University of Twente, from the MESA+ Institute for Nanotechnology collaborating with scientists of the Max Planck Institute of Colloids and Interfaces in Golm, Germany. | |
| Dr. Vancso explained his team's findings to Nanowerk: "We made micro- and nanocontainers, using special polymers, containing iron in their main chain, which respond reversibly and alter their number of electrons to changes triggered by oxidation and reduction using chemical agents. Upon changes of the number of electrons on iron atoms in the polymer chains, which make up the wall of the nanocontainers, the walls can be made either open (permeable), or closed (non-permeable) for other molecules which would move into the containers or out of the containers, depending on the previous loading of the containers." | |
| ‘Smart’ polymers can recognize a stimulus as a signal and then significantly alter for instance their chain conformation in response to small changes in the environmental conditions. Researchers are very interested in molecular structures that are composed of such materials, with the ability to be triggered to contract or expand in a controlled fashion. | |
| Conventional, organic polyelectrolyte-based microcapsules have limitations in some significant applications due to their slow response to trace amounts of trigger and restrictions on the choice of stimuli. Scientists found that electrochemical stimuli are very promising, though they requires the construction of capsules with redox-active compounds. | |
| Vancso and his collaborators used water soluble PFS - poly(ferrocenylsilanes) - polycations and polyanions. These compounds belong to the rare class of main-chain organometallic polyelectrolytes, which have recently been reported. They all bear certain charges on the polymer side groups so that they can be used in the electrostatic layer-by-layer (LBL) self-assembly process to form multilayer films and hollow capsules with defined structure and function due to the molecular characteristics of the organometallic main chain. | |
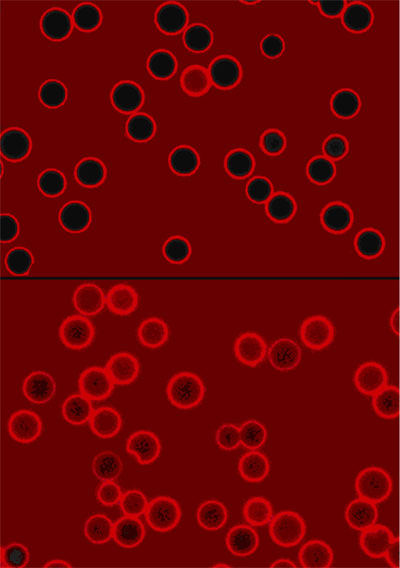 |
Confocal fluorescence microscopy images of empty (top) and loaded (bottom) PFS capsules. (Copyright by Y. Ma, W.F. Dong, M.A. Hempenius, H. Moehwald, G.J. Vancso; published with permission.) |
| The team, including Ms. Yujie Ma, Ph.D. student, and Dr. Mark Hempenius, UD, from Twente, and Dr. Wen-Fei Dong and Professor Helmuth Möhwald, of Golm, published their results in the September issue of Nature Materials ("Redox-controlled molecular permeability of composite-wall microcapsules"). | |
| Vancso explains that the polymer chains that make up the wall of the containers are assembled layer-by-layer, using positively, as well as negatively charged polymers. These electrostatic charges hold the polymer chains together. | |
| "The polymer wall, however, looks like rose petals, or onion sheets, consisting of layers of polymer molecules" he says. "This shell surrounds the interior of the container, which is filled either by a solvent, or a solvent with a few captive molecules. If large molecules are added to the water surroundings of the capsules, these added molecules may, or may not penetrate into the capsules depending on the number of electrons within the polymer chains encompassed by the capsule wall." | |
| The number of electronic charges in the wall, and thus the interaction among the polymer chains, can be controlled reversibly and precisely, by adding oxidants, or reducing agents, to the surrounding medium. | |
| Thus the flow of the „inmate” molecules in and out of the container can be controlled, by opening or closing the pores of the wall upon addition of suitable chemical agents. | |
| "The fundamental ingredient of these capsule walls is the redox active polymer chains including iron, which allowed the application of oxidants, or reducing agents for commanding permeability for the first time" says Vancso. As numerous biochemical processes, which take place in water, are accompanied by oxidation, or reduction, these smart container capsules hold the promise of biomedical and biological use, and have application potential in other „green” areas such as encapsulation and release of food additives, drugs, cosmetic agents, etc." | |
| Other uses may play a role in fundamental science such as in bionanochemistry, e.g. to encapsulate (and protect) single enzyme catalyst molecules and monitor their reaction in confinement with other molecules within the nanocontainers. If large number of containers are used simultaneously, and addressed individually, synthetic or natural catalysts may be compared and selected for optimum efficiency using the cages as nanoreactor vessels in fast, parallel, so-called high throughput combinatorial chemistry experiments. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com.
