| Posted: Oct 17, 2011 | |
Autonomous nanomotor based on a true nanobattery |
|
| (Nanowerk Spotlight) The catalytic conversion of chemical to mechanical energy, which is ubiquitous in biological systems, also is the basis for many of the engine systems that nanotechnology researchers are developing. Catalytic 'engines' will be key components of active micron- and sub-micron scale systems for controlled movement, particle assembly, and separations (see "Catalytic nanotransporters for nanotechnology applications outside biological systems"). | |
| So far, most of these catalytic micro- and nanomotors use hydrogen peroxide as the fuel. The major problem associated with this is that the produced oxygen bubbles make the observation and detailed study of these motors difficult. Researchers at the Pennsylvania State University have now introduced a new bubble-free, high efficient nanomotor system that involves the operation of a miniaturized copper-platinum nanobattery. | |
| "We have discovered that a short-circuited nanobattery can be moved by self-electrophoresis resulting from oxidation and reduction occurring, respectively, at the two metals," Ran Liu, a Ph.D. researcher in Ayusman Sen's group at Penn State, tells Nanowerk. "The generated current can be directly converted to mechanical force." | |
 |
|
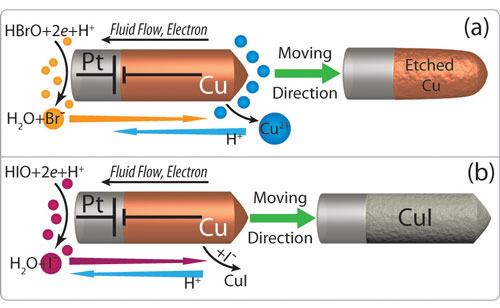
| Mechanism of self-powered Cu-Pt nanomotor motion in (a) aq. bromine and (b) aq. iodine. (Reprinted with permission from American Chemical Society) | |
| The team reports their findings in the September 30, 2011 online edition of Journal of the American Chemical Society (JACS) ("Autonomous Nanomotor Based on Copper–Platinum Segmented Nanobattery"). In this paper, first-authored by Liu, they demonstrate that this motor system is significantly more efficient than the previously described bimetallic systems, such as platinum-gold segmented nanorod in hydrogen peroxide. | |
| "In the latter case, only a small fraction of the hydrogen peroxide fuel was utilized for generation of electrochemical current while the rest of it is wasted due to its rapid catalytic decomposition on the platinum-end alone," says Sen. "Our study confirms the generality of self-electrophoresis as a mechanism for micro/nanomotor movement and suggest that virtually any redox reaction occurring asymmetrically on an appropriate micro/nanostructure can be employed in the design of self-powered systems." | |
| The nanomotor described by Liu and Sen is based on self-propelling of template-synthesized copper-platinum bimetallic nanowires in either bromine or iodine diluted solutions. The motion is due to self-electrophoresis induced by the redox reaction occurring at the two electrodes of the copper-platinum nanobattery. | |
| Cu-Pt nanobatteries moving in 0.25 mM iodine solution. The calculated velocity is around 12 micron/s. (With permission from American Chemical Society) | |
| As the researchers point out, their work serves to underline self-electrophoresis as a generally applicable propulsion mechanism for micro/nanoscale objects and significantly expands the range of redox reactions that can be employed for this purpose. | |
| For example, other metal pairs and fuels can be employed for the design of nanobattery-based motors. | |
| Apart from the propulsion aspect of these research findings, this work also marks the fabrication of one of the world's smallest nanobatteries on a single copper-platinum segmented nanorod. | |
| "The concept of nanobattery already existed, but most of these so-called 'nanobatteries' are actually referring to the nanostructured electrodes used in a battery," says Liu. "However, in this study, we shrink the whole battery to nano-size by fabricating Cu-Pt nanorods in the nanopores of the alumina template via the approach of sequential electrodeposition copper and platinum." | |
| Sen notes that, although the fuels utilized in the present study have the virtue of high efficiency and the system is bubble-free, further effort is required to find more environmentally friendly, and especially biocompatible, fuel systems. | |
| "An additional challenge is the design of moving micro/nanobatteries that can be recharged and used repeatedly" he describes another aspect of the team's future research areas. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
