| Posted: Mar 12, 2014 | |
Making cells 'throw up' nanomaterials to rescue them from apoptosis |
|
| (Nanowerk Spotlight) The investigation of effects of engineered nanomaterials on endothelial cells – which form the inner lining of blood vessels – is a critical safety issue. Already, various engineered nanomaterials are being designed for biomedical applications for intravascular use and other nanomaterials may reach the vasculature as a result of occupational, environmental, or other types of exposure. Endothelial dysfunctions leads to life-threatening vascular disorders, including hypoperfusion and organ damage, and thrombotic and/or bleeding pathologies. | |
| It was previously described that various nanomaterials have activating effects on cell autophagy or inhibit the autophagic flux ("Autophagy and lysosomal dysfunction as emerging mechanisms of nanomaterial toxicity"). However, the mechanism underlying the effects of carboxylated carbon nanotubes (CNTs) on vascular endothelial cells has not been studied. | |
| An international team of scientists U.S. Food and Drug Administration (FDA), the National Institute of Standards and Technology (NIST), and Charles University in Prague and Academy of Sciences of the Czech Republic, has now demonstrated than carboxylated multi-walled CNTs (MWCNTs) in contrast to their pristine counterparts induce autophagosome accumulation by inhibiting the autophagic flux. The team, led by Jan Simak, Ph.D., a Principal Investigator at the FDA's Center for Biologics Evaluation and Research (CBER), has reported their findings in the February 24, 2014, online edition of Nanomedicine ("Toxicity of carboxylated carbon nanotubes in endothelial cells is attenuated by stimulation of the autophagic flux with the release of nanomaterial in autophagic vesicles"). | |
| In this article, the researchers elucidated the mechanism of cytotoxicity of carboxylated MWCNTs on cultured endothelial cells and they show a new potential way of pharmacological cytoprotection against cytotoxic effect of carboxylated MWCNTs. | |
| Autophagy – macroautophagy, “self-eating” – is a degradation pathway in which cytoplasmic content including abnormal proteins, damaged organelles, invading microorganism or foreigner materials are engulfed in a spherical double membrane structures called autophagosomes and degraded by lysosomes. | |
| Autophagy is essential for cells to break down their own components; however, when dysregulated either by inhibition or activation, it leads to cell injury and death. | |
| "Carboxylated MWCNTs exhibit cytotoxicity in cultured human umbilical vein endothelial cells (HUVECs) associated with the profound accumulation of autophagosomes," Simak tells Nanowerk. "By investigating different molecular markers of autophagy, we found that the autophagosome accumulation induced by carboxylated MWCNTs is caused by blockade of the autophagic flux rather than by activation of autophagy. It means that cells are filled with nanomaterial containing autophagosomes, which are not further processed, and that leads to cell death by apoptosis." | |
| "Interestingly" he continues, "we found that stimulation of the autophagic flux with 1 nmol/L bafilomycin A1 attenuates the cytotoxicity of carboxylated MWCNTs in HUVECs with the extracellular release of the nanomaterial in autophagic microvesicles." | |
 |
|
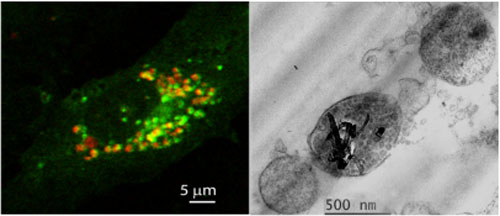
| Laser scanning confocal microscopy (left) shows accumulation of autophagosomes (green) containing carboxylated MWCNTs (red) in endothelial cells (HUVEC). Transmission electron microscopy (right) shows autophagic vesicles containing carboxylated MWCNTs released from HUVEC after stimulation with 1nM bafilomycin A1 (Image: Silvia De Paoli, FDA) | |
| These findings are significant because understanding of the vascular toxic effects of CNTs is critical for evaluation of the widely discussed health risks of CNTs during environmental, occupational and biomedical exposure. | |
| In addition, this study developed a novel strategy for pharmacological modulation of the cellular adverse effects of nanomaterials to optimize their biocompatibility for therapeutic and diagnostic applications. | |
| "Our initial hypothesis was that the extracellular release of impaired autophagosomes containing nanomaterial would be a reasonable strategy for cell survival,"explains Martina Orecna, a research fellow at CBER and the paper's first author. "However, very little is known about the mechanism underlying the exocytosis of autophagic vesicles and the release of nanomaterials in autophagic vesicles has not been described thus far." | |
| Carboxylated MWCNTs agglomerate in blood plasma as well as in the culture medium and readily bind serum proteins, resulting in the formation of CNT-protein aggregates. Protein misfolding occurs in this process. | |
| Therefore, the researchers speculated that the intracellular accumulation of carboxylated MWCNTs is somewhat analogous to the accumulation of misfolded proteins in neuronal cells observed in neurodegenerative proteinopathies, such as Alzheimer’s disease, Huntington’s disease or Parkinson’s disease. | |
| Bafilomycin A1 is a macrolide antibiotic which exhibits differential, concentration-dependent effects on autophagy. Although the cytoprotective role of a low dose of bafilomycine A1 in attenuating the toxicity of foreign materials has not been studied previously, such a function has been observed in certain proteinopathies. | |
| In this present work, the team has now demonstrated that stimulation of the autophagic flux by 1 nmol/L bafilomycin A1 attenuates the cytotoxicity induced by carboxylated MWCNTs in cultured endothelial cells with the extracellular release of the nanomaterial in autophagic vesicles. | |
| This finding opens a new way for pharmacological cytoprotection against toxic effects of nanomaterials. | |
| "Our report is a mechanistic in vitro study which needs follow-up investigations on several important issues," Simak notes. "Further studies in different types of endothelial cells and in other cell types are needed to assess the specificity or general character of the observed responses." | |
| Also, the study was performed using carboxylated MWCNTs surface modified in our laboratory. A panel of various nanomaterials, including CNTs with different grade of surface modification need to be tested. | |
| Among several factors, dispersion of the nanomaterials indicated by size distribution of the nanomaterial-protein agglomerates impacts a choice of the mechanism of cellular uptake including effect on cell autophagy. | |
| Simak points out that it is likely that only namomaterial-protein agglomerates of a specific size range cause accumulation of autophagosomes. "Therefore cytoprotective effect of low concentration of bafilomycin A1 works under specific conditions needs to be fully investigated." | |
| Translation of the in vitro findings to in vivo model is another challenging task. In vivo animal studies will tell more about potential applications of these findings, such as therapeutic stimulation of autophagic flux after intoxications with nanomaterial or prevention of vascular adverse effects in medical applications of certain nanomaterials used in diagnostic biosensors, drug delivery nanosystems, imaging nanoprobes for intravascular use and other devices that come in contact with blood. | |
| "Based on our novel findings, molecular targets of the low dose bafilomycin A1 warrant further investigation," concludes Simak. "Also, the molecular mechanism of the exocytosis of autophagic vesicles in endothelial cells remains to be elucidated." | |
| "We need to understand how CNTs interact with other blood components and with vessel wall," he says. "The next step is to investigate how surface modifications of CNTs modulate their blood and vascular biocompatibility. The role of plasma protein corona on nanomaterials in contact with blood as well as effects of nanomaterial-protein agglomerates in evaluation of nanomaterial biocompatibility in vitro and in vivo need to be investigated." | |
| Disclaimer: The findings and conclusions in this article have not been formally disseminated by the Food and Drug Administration and should not be construed to represent any Agency determination or policy. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
