| Posted: Jun 25, 2014 | |
Untethered active microgripper for single-cell analysis |
|
| (Nanowerk Spotlight) There are a wide range of passive devices such as beads, wells and tubes that can be used to capture and confine single cells. Previous active cell grippers with moving parts have relied on electrical modalities which can be challenging to implement off-chip and in a highly parallel manner (see for instance: "A gripping tale for nanomanufacturing"). | |
| Researchers have now, for the first time, demonstrated an untethered active microgripper that can be used to capture and contain single cells. These single-cell grippers, made from biocompatible and bioresorbable materials, derive their actuation energy from thin-film stresses. They can be arrayed for high throughput in vitro assays and imaging. | |
| Since their operation does not need any kind of wires or batteries, they can be released for use as untethered tools. | |
| "We employed varying sizes of these grippers to capture individual fibroblasts and red blood cells," David H. Gracias, a professor at Johns Hopkins University, tells Nanowerk. "These cells were alive and could be assayed or fixed for imaging. Because these devices are fabricated in 2D and subsequently folded into 3D, future studies could explore patterned topography such as spikes, holes, nanoscale roughness, and biochemical surface functionalization in specific designs onto one or more device walls." | |
| Gracias and his team published their findings in the June 10, 2014 online edition of Nano Letters ("Self-Folding Single Cell Grippers"). | |
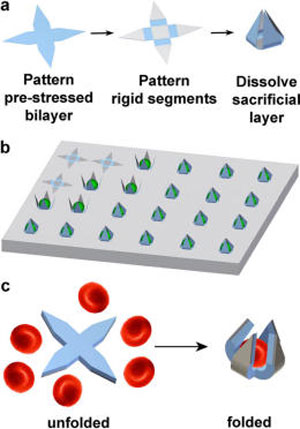 |
|
| Illustration of single cell gripper fabrication and use on substrates or as untethered tools. (a) Fabrication scheme for creating single cell grippers. The prestressed actuator hinge is a SiO/SiO2 bilayer, while the rigid segments are made of SiO. Upon dissolution of the sacrificial layer, the arms are released and self-actuate to close around cells. An optional thermoresponsive trigger layer can be molded atop the grippers. (b) Illustration of cells captured by single cell microgrippers arrays. (c) Illustration of untethered single cell grippers and red blood cell capture. (Reprinted by permission of American Chemical Society) | |
| The researchers note that their approach is inspired by previous studies on the stress-based roll-up and self-folding of thin films (read our previous Nanowerk Spotlight: "Stress is good for nanofabrication") and the energy required to enable gripper motion is derived from the differential residual stress in nanoscale bilayers. | |
| "Our approach utilizes photolithography, which is a high throughput technique capable of fabricating 500 000 to 10 million single grippers on a 3 inch wafer or potentially over 100 million on a 12 inch wafer which is the size of wafers used currently in CMOS fabrication facilities," explains Kate Malachowski, a graduate student in Gracias' group and lead author of the paper. "Additionally, the grippers can be actuated to close around single cells en masse, creating devices with patterns in all three dimensions." | |
| The team developed several gripper variants with three or four arms, varying in size from 10 to 70 µm in length (tip-to-tip when open), which is an appropriate size range to grasp a variety of individual cells. | |
| Since the grippers can be manufactured en masse and also operate autonomous, they can be utilized to capture and analyze single cells in vivo in a high throughput manner. | |
| Additionally, since the process is compatible with conventional microfabrication, the surfaces of the devices can be patterned with electrical or optical biosensors to enable real time single cell analysis after capture. | |
| On the in vivo side, the scientists anticipate that single cell grippers could be utilized to capture and possibly analyze single cells within hard to reach places in the human body. These capabilities are enabled by the composition of materials that can be bioresorbable allowing for clearance from the body by dissolution, the small size that enables access to small conduits, and the absence of any wires or tethers allowing them to be deployed and operated in a highly parallel manner. | |
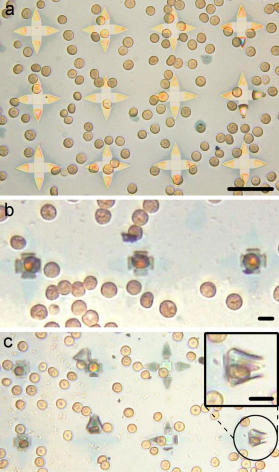 |
|
| Capture of single red blood cells and untethered single cell grippers. (a-c) Optical images of red blood cells trapped in 35 µm SiO/SiO2 grippers. (a) Grippers with red blood cells prior to folding and release from the substrate. Scale bar is 35 µm. (b-c) Red blood cells captured by the grippers. Scale bars are 10 µm. (Reprinted by permission of American Chemical Society) | |
| Gracias cautions that there still remain numerous challenges before these grippers could see real-world applications: "On the in vitro side, we are still challenged by the integration of biosensing elements on the surfaces of the devices; less than perfect yields; and cell viability especially for real time monitoring over prolonged durations. For in vivo applications, there are a huge number of challenges including guidance; introduction into the body; retrieval; site specific or targeted actuation; and patient safety." | |
| Previously, Gracias' has shown that larger untethered grippers could be used to biopsy gastrointestinal organs ("Team deploys hundreds of tiny untethered surgical tools in first animal biopsies") suggesting that the realization of this concept is within reach. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
