| Posted: Jul 03, 2014 | |
Biomimetic nano-environments as templates for skin regeneration |
|
| (Nanowerk Spotlight) Cellular functions within living organisms are extremely complex processes and researchers have been using nanopatterned substrates to control and monitor cellular functions in order to design and fabricate nanoscale biotechnological systems. Especially stem cell research has benefitted from nanopatterned surfaces to maintains stem cells' long-term viability and phenotype during experiments. Nevertheless, despite the intense scientific efforts to achieve precise control of stem cell fates with engineered nanopatterned substrates, reliable and cost effective control of stem cell behavior remains a challenge. | |
| Most of the tissues and organs in the human body, with their distinct three-dimensional structures, require support – scaffold/substrate, template, and artificial extracellular matrix or niche – for their formation from diverse cells. | |
| Researchers have now fabricated biomimetic substrates that are similar to that of the native extracellular matrix (ECM) in the epidermis which assists proliferation, differentiation, and biosynthesis of the keratinocyte (i.e. human outer skin) cells. | |
| "Although sophisticated technologies and protocols have been employed for the fabrication of chemical functional substrates, our proposed methodology is simple and reproducible and, thus, may have good potential to be used in commercial processes," Morteza Mahmoudi, a professor at Tehran University of Medical Sciences, who heads the Laboratory of Nano-Bio Interactions, explains to Nanowerk. "In this work, we have introduced a shape-/pattern-induced stem cell differentiation phenomenon as an overlooked-factor in the substrate/scaffold designing procedure, which may represent a new concept on regeneration of skins from cells with the support of biomaterials with no chemical agents (e.g., growth factors)." | |
 |
|
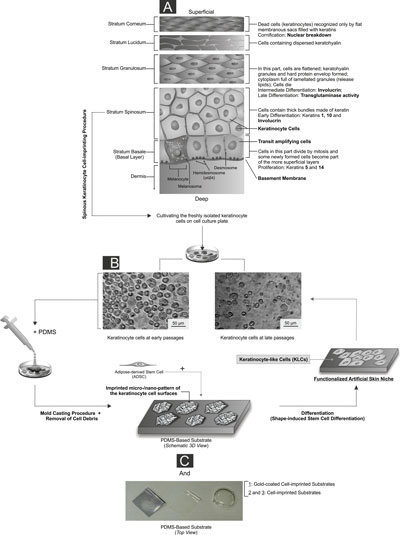
| The detailed architecture of the basal-to-spinous layer transition together with illustration of the experimental set-up and artificial skin niche study steps. (click on image to enlarge) A) The program of epidermal differentiation is illustrated in this schematic, exposing the basement membrane at the base, the proliferative basal layer, and the four differentiation stages: stratum spinosum layer, stratum granulosum layer, stratum lucidum layer and outermost stratum corneum. The key molecular markers which are shown in this schematic are described in detail in the paper. The proliferative basal cells, as a multipotent progenitor of the epidermis layer, adhere to an underlying basement membrane (separating the dermis from the epidermis), and can differentiate into the spinous cells in the suprabasal layer. Quiescent human epidermal stem cells and their transient amplifying cell progeny give rise to a column of differentiated keratinocytes in diverse layers was depicted. The transit-amplifying cells constantly produce progeny which move upward as they terminally differentiate and are ultimately lost from the skin surface (superficial). B) The cultivated keratinocyte cells on different well plates at different passage levels are shown in this figure. C) In order to fabricate the PDMS-based substrate which mimics the natural stratum spinosum layer’s function, the keratinocyte cells were grown on a cell culture plate and their complete morphologies at different stages were transferred to a silicone replica by a mold casting procedure. After the removal of damaged cells and cell debris from the substrates, a negative PDMS-based replica (i.e., non-coated and gold coated heterostructure substrates with varied thicknesses) with an imprinted pattern of the cell surfaces was achieved. This unique structure was utilized as an efficient platform to manipulate the stem cells in order to achieve production of the keratinocyte-like cells. (Reprinted with permission from American Chemical Society) (click on image to enlarge) | |
| Mahmoudi and his collaborators have published their findings as a 'Just Accepted Manuscript' in the June 26, 2014 online edition of ACS Applied Materials & Interfaces ("Cell-Imprinted Substrates Act as Artificial Niche for Skin Regeneration"). | |
| Initially, the researchers hypothesized that induction of specific mature adult cell shapes to stem cells – which were isolated from different sources – can result in differentiation of stem cells to specific mature cells. In their current work, they have focused on skin, as a classical example of a tissue, which is supported by stem cells; the skin needs to be regenerated constantly during normal homeostasis and after wounding. | |
| "In this context, we have found that a 3D surface imitating the morphology of the keratinocyte plasma membrane could be used as a biomimetic template for differentiation of adipose-derived stem cells (ADSCs) into keratinocyte-like cells (KLCs)," says Mahmoudi. "It is worth noting that our artificial biomimetic micro/nano-environments were fabricated by a cell-imprinted procedure based on mature human keratinocyte morphological templates." | |
| Since the shape and geometry of the cell nucleus could alter the gene expression patterns, the researchers utilized a molecular dynamics approach to probe the effect of confining geometry on the chain arrangement of simulated chromatin fibers in the stem cell nucleus. | |
| As they point out, the obtained data could imply an obvious role of direct mechanotransduction to the nucleus and, consequently, to biochemical mechanotransduction. | |
| The main goal of this research is to design a novel cell-based therapeutical technology that results in efficient medical treatments – e.g., tissue-engineered skin substitutes – for diseases. | |
| "Based on the obtained results, one can expect that our cell-imprinted substrates might pave the way for a reliable, efficient, and cheap controlling of stem cell differentiation toward skin cells for wound healing and skin tissue engineering applications," says Mahmoudi. | |
| The team found that, besides the cells themselves and external conditions, the physico-mechanical properties of the substrates are very important factors for controlling stem cell fate. As a result, the physico-mechanical properties of the native niche – such as adhesivity, stiffness, and topography – for different types of stem cells should be considered by researchers in their biomimetic approaches fabrication. | |
| "Our work proves conclusively that stem cell function does follow form," notes Mahmoudi. "Make a stem cell look like you want it to act like – and it does so." | |
| The artificial niche introduced by this work may ultimately lead to advances in tissue engineering towards implants integrating bioprinting, nano-biosensors and advanced biomaterials. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
