| Posted: Jun 26, 2008 | |
Nanotechnology miniaturization could lead to a Lab-on-a-CNT |
|
| (Nanowerk Spotlight) Carbon nanotubes (CNTs) have been widely used as electrodes for chemical and biological sensing applications and many other electrochemical studies. With their unique one-dimensional molecular geometry of a large surface area coupled with their excellent electrical properties, CNTs have become important materials for the molecular engineering of electrode surfaces where the development of electrochemical devices with region-specific electron-transfer capabilities is of paramount importance. | |
| It has been demonstrated that carbon nanotubes enhance the electrochemical activity of biomolecules and promote the electron-transfer reactions of redox proteins, such as myoglobin, cyctochrome c, and microperoxidase MP-11. The enhanced electrochemical activity and electron transfer rate at CNT electrodes have been widely believed to arise from the nanotube tips. However, no convincing experimental evidence has been obtained to prove that the CNT tip is more electrochemically active than its sidewall. | |
| Contradicting this common belief, researchers have now found that, surprisingly, the electrochemistry at carbon nanotube electrodes is not always facilitated by the nanotube tip. In fact, the relative electrochemical sensitivity of the nanotube tip and sidewall varies for different electrochemical probes proceeding with different reaction mechanisms. | |
| These new findings could not only provide insights for fundamental understanding of electrochemical processes taking place at the CNT electrodes, but also facilitate the design and development of novel CNT-based electrodes for various potential applications, ranging from chemical and bio-sensors to energy conversion devices. | |
| Knowing that the CNT tip and sidewall have different electrochemical reactivity to different chemical species, it might even be possible to build sensor chips on a single carbon nanotube. | |
| "Although carbon nanotubes have been widely investigated as electrodes in various electrochemical systems, nonaligned carbon nanotubes or relatively short aligned nanotubes with at most a few hundreds microns in length were used in almost all of the previous studies," Dr. Liming Dai explains to Nanowerk. "The synthesis of super-long, i.e. millimeter scale, vertically-aligned carbon nanotubes has only been a recent development. In cases that involve conventional randomly-orientated nanotubes or relatively short aligned nanotubes as electrodes it is difficult to distinguish the electrochemical role of the nanotube tip from its sidewall, or vice versa." | |
| Dai, Wright Brothers Institute Endowed Chair in Nanomaterial at the University of Dayton, Ohio, and his group have now demonstrated experimentally the effects of the nanotube tip and sidewall and their oxidation states on the electrochemistry of various commonly used electrochemical probes at CNT electrodes. The results have been published in the June 13, 2008 online edition of Angewandte Chemie International Edition ("Electrochemistry at Carbon Nanotube Electrodes: Is the Nanotube Tip More Active Than the Sidewall?"). | |
| Prior to this paper, there was no convincing experimental evidence to either prove or disprove the common belief that the carbon nanotube tip is more electrochemically reactive than it sidewall. | |
| Dai mentions that his group has long been working on the synthesis and functionalization of vertically-aligned carbon nanotubes for various applications, including CNT chemical and bio-sensors. They have previously developed polymer-masking technologies for region-specific functionalization of aligned carbon nanotubes. The recent growth of the super-long vertically-aligned carbon nanotubes by Dai's group provided them with a unique opportunity to solve the question concerning the role of nanotube tip and sidewall in the electrochemistry at CNT electrodes. | |
 |
|
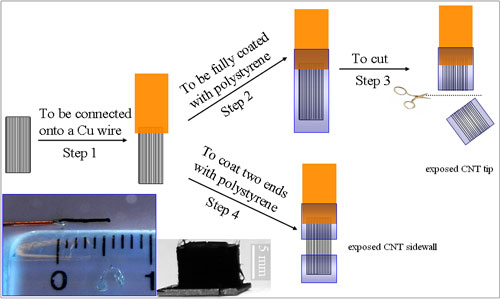
| A schematic representation of the procedure for preparing the CNT electrodes with only the nanotube tip or sidewall accessible to electrolyte. Inset shows a digital photograph of (right panel) the as-synthesized aligned superlong CNTs and (left panel) a nanotube electrode thus prepared with an aligned super-long CNT bundle connected to a copper wire. (Adopted from Wiley-VCH Verlag) | |
| "By using superlong (∼5 mm) vertically aligned carbon nanotube bundles selectively masked with a nonconducting polymer coating at the nanotube sidewall or tip(s), we have successfully limited the electrolyte access to the nanotube tip or sidewall only for region-specific electron transfer between the electrochemical probes and the CNT electrodes," says Dai. "Surprisingly, we found that the electrochemistry at carbon nanotube electrodes is not always facilitated by the nanotube tip and/or oxygen-containing surface groups. In fact, the relative electrosensitivity to the nanotube tip and sidewall and their oxidation states varies with different electrochemical probes and relates to distinct reaction mechanisms." | |
| The researchers point out that further experiments are needed in order to fully understand the governing principles for the influence of the CNT tip/sidewall on the electron transfer rate of various electrochemical probes at the CNT electrodes. Nevertheless, these findings should facilitate the design and development of novel CNT-based electrodes of practical significance. For instance, they might allow researchers to region-specifically functionalize individual carbon nanotube electrodes for multifunctional applications. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
