Showing Spotlights 25 - 32 of 44 in category All (newest first):
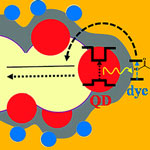 While the dye sensitized photovoltaic cell is a fairly mature design, researchers are still trying to improve its efficiency with various techniques, including structuring nanoporous electrodes to provide higher surface area and better charge transport, replacement of the liquid electrolyte by a solid one in order to prevent the electrolyte evaporation, and ways to widen the narrow absorption spectra of molecular dyes. In a standard DSSC, an organic molecule adsorbed on the surface of a porous electrode absorbs light and then initiates the charge separation process eventually leading to generation of photocurrent. One major difficulty in such cells is that very few dyes can absorb a broad spectral range, essentially covering the solar spectrum. In contrast, broad spectral coverage is an inherent property of semiconductor nanocrystals. The latter, however, turn out to do a rather lousy job in separating the charges. Researchers in Israel have now presented a new configuration for quantum dot sensitized DSSCs via a FRET process.
While the dye sensitized photovoltaic cell is a fairly mature design, researchers are still trying to improve its efficiency with various techniques, including structuring nanoporous electrodes to provide higher surface area and better charge transport, replacement of the liquid electrolyte by a solid one in order to prevent the electrolyte evaporation, and ways to widen the narrow absorption spectra of molecular dyes. In a standard DSSC, an organic molecule adsorbed on the surface of a porous electrode absorbs light and then initiates the charge separation process eventually leading to generation of photocurrent. One major difficulty in such cells is that very few dyes can absorb a broad spectral range, essentially covering the solar spectrum. In contrast, broad spectral coverage is an inherent property of semiconductor nanocrystals. The latter, however, turn out to do a rather lousy job in separating the charges. Researchers in Israel have now presented a new configuration for quantum dot sensitized DSSCs via a FRET process.
Feb 23rd, 2010
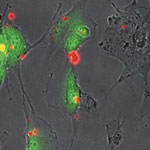 Oncogenes are genes that are associated with the development of cancer - when mutated or expressed at high levels, they can help turn a normal cell into a tumor cell. Promising new chemotherapeutic strategies have therefore focused on suppressing oncogenes. One such approach is based on RNA interference (RNAi), a technique wherein small double-stranded RNA molecules can sequence-specifically inhibit the expression of targeted oncogenes. The idea here is that with the help of small interfering RNA (siRNA), key oncogenes that modulate signaling pathways and thereby regulate the behavior of malignant tumor cells can be manipulated. To harness the full potential of this approach, the prime requirements are to deliver the siRNA molecules with high selectivity and efficiency into tumor cells and to monitor both siRNA delivery and the resulting knockdown effects at the single-cell level.
Oncogenes are genes that are associated with the development of cancer - when mutated or expressed at high levels, they can help turn a normal cell into a tumor cell. Promising new chemotherapeutic strategies have therefore focused on suppressing oncogenes. One such approach is based on RNA interference (RNAi), a technique wherein small double-stranded RNA molecules can sequence-specifically inhibit the expression of targeted oncogenes. The idea here is that with the help of small interfering RNA (siRNA), key oncogenes that modulate signaling pathways and thereby regulate the behavior of malignant tumor cells can be manipulated. To harness the full potential of this approach, the prime requirements are to deliver the siRNA molecules with high selectivity and efficiency into tumor cells and to monitor both siRNA delivery and the resulting knockdown effects at the single-cell level.
Jan 19th, 2010
 The usefulness of quantum dots comes from their peak emission frequency's extreme sensitivity to both the dot's size and composition. QDs have been touted as possible replacements for organic dyes in the imaging of biological systems, due to their excellent fluorescent properties, good chemical stability, broad excitation ranges and high photobleaching thresholds. In order for quantum dots to be useful as as nanoemitters for biological imaging, they need to be linked with a specific sensor molecule that exclusively targets a biomolecule of interest. These conjugations are usually made using small linker molecules although this often demands multistep procedures and may suffer from QD colloidal instabilities during the coupling reactions. Demonstrating hyaluronic acid-QD conjugates, researchers in Korea have now solved this problem by simple electrostatic conjugation which offers stable and size-tunable conjugates.
The usefulness of quantum dots comes from their peak emission frequency's extreme sensitivity to both the dot's size and composition. QDs have been touted as possible replacements for organic dyes in the imaging of biological systems, due to their excellent fluorescent properties, good chemical stability, broad excitation ranges and high photobleaching thresholds. In order for quantum dots to be useful as as nanoemitters for biological imaging, they need to be linked with a specific sensor molecule that exclusively targets a biomolecule of interest. These conjugations are usually made using small linker molecules although this often demands multistep procedures and may suffer from QD colloidal instabilities during the coupling reactions. Demonstrating hyaluronic acid-QD conjugates, researchers in Korea have now solved this problem by simple electrostatic conjugation which offers stable and size-tunable conjugates.
Jul 7th, 2009
 In a recent Nanowerk Spotlight we reported on a single molecule approach to directly visualize and map protein binding sites on DNA using fluorescent quantum dots. One of the challenges the researchers in this work had was to measure distances between probes bound to combed DNA with nanometer resolution. Whereas very short distance (below 10 nm) can be assessed by FRET measurements and distances above the Rayleigh criterion can be measured, say, with a standard microscopy picture and a ruler, distances in between need to be addressed differently. This is were a novel approach by scientists at UCLA fills the gap, and, as they claim, better than other techniques do.
In a recent Nanowerk Spotlight we reported on a single molecule approach to directly visualize and map protein binding sites on DNA using fluorescent quantum dots. One of the challenges the researchers in this work had was to measure distances between probes bound to combed DNA with nanometer resolution. Whereas very short distance (below 10 nm) can be assessed by FRET measurements and distances above the Rayleigh criterion can be measured, say, with a standard microscopy picture and a ruler, distances in between need to be addressed differently. This is were a novel approach by scientists at UCLA fills the gap, and, as they claim, better than other techniques do.
May 27th, 2009
 Proteins that bind to specific sites of DNA are essential to all biological functions of DNA. These DNA-binding proteins include transcription factors which modulate the process of transcription, various polymerases, nucleases which cleave DNA molecules, and histones which are involved in chromosome packaging in the cell nucleus. Developing methods to precisely determine the locations and occupancy of DNA-binding proteins is instrumental to scientists' understanding of cellular processes like gene expression and regulation. Motivated by the desire to overcome some of the inherent limitations of existing biochemical techniques for mapping protein binding sites on DNA, scientists at UCLA have now demonstrated the viability of a single molecule approach to directly visualize and map protein binding sites on DNA using fluorescent quantum dots, allowing multicolor, nanometer-resolution localization.
Proteins that bind to specific sites of DNA are essential to all biological functions of DNA. These DNA-binding proteins include transcription factors which modulate the process of transcription, various polymerases, nucleases which cleave DNA molecules, and histones which are involved in chromosome packaging in the cell nucleus. Developing methods to precisely determine the locations and occupancy of DNA-binding proteins is instrumental to scientists' understanding of cellular processes like gene expression and regulation. Motivated by the desire to overcome some of the inherent limitations of existing biochemical techniques for mapping protein binding sites on DNA, scientists at UCLA have now demonstrated the viability of a single molecule approach to directly visualize and map protein binding sites on DNA using fluorescent quantum dots, allowing multicolor, nanometer-resolution localization.
May 19th, 2009
 The properties of a quantum dot are not only determined by its size but also by its shape, composition, and structure, for instance if it's solid or hollow. A reliable manufacturing technology that makes use of nanocrystals' properties - for a wide-ranging number of applications in such areas as catalysis, electronics, photonics, information storage, imaging, medicine, or sensing - needs to be capable of churning out large quantities of nanocrystals where each batch is produced according to the exactly same parameters. In a recent review article, Sara Skrabalak from Indiana University and Younan Xia from Washington University in St. Louis, describe recent advances in seeded growth as the ultimate approach to producing metal nanocrystals with precisely controlled sizes, shapes, and compositions - the necessary first step toward their use and assembly for large-scale applications.
The properties of a quantum dot are not only determined by its size but also by its shape, composition, and structure, for instance if it's solid or hollow. A reliable manufacturing technology that makes use of nanocrystals' properties - for a wide-ranging number of applications in such areas as catalysis, electronics, photonics, information storage, imaging, medicine, or sensing - needs to be capable of churning out large quantities of nanocrystals where each batch is produced according to the exactly same parameters. In a recent review article, Sara Skrabalak from Indiana University and Younan Xia from Washington University in St. Louis, describe recent advances in seeded growth as the ultimate approach to producing metal nanocrystals with precisely controlled sizes, shapes, and compositions - the necessary first step toward their use and assembly for large-scale applications.
Feb 2nd, 2009
 Quantum dots are emerging as an important class of nanoparticles with applications ranging from medicine to energy. These nanocrystals possess size tunable optical and electronic properties resulting from quantum confinement which allow them to be suitable candidates for applications in solar cells and light emitting devices. For instance, quantum dots have been identified as important light harvesting material for building highly efficient solar cells - when exposed to light at certain wavelengths they can generate free electrons and create an electrical current. Having a high resistance to photobleaching, quantum dots (QDs) also are attractive materials for optoelectronics and in vivo biosensing. Researchers have now demonstrated that QDs, in addition to being excellent fluorescent probes, can be used as photoacoustic (which combines the advantages of optical absorption contrast with ultrasonic spatial resolution for deep imaging) and photothermal contrast agents and sensitizers, thereby providing an opportunity for multimodal high resolution photoacoustic/photothermal-fluorescent imaging as well as sensitizers in nanotherapeutic applications.
Quantum dots are emerging as an important class of nanoparticles with applications ranging from medicine to energy. These nanocrystals possess size tunable optical and electronic properties resulting from quantum confinement which allow them to be suitable candidates for applications in solar cells and light emitting devices. For instance, quantum dots have been identified as important light harvesting material for building highly efficient solar cells - when exposed to light at certain wavelengths they can generate free electrons and create an electrical current. Having a high resistance to photobleaching, quantum dots (QDs) also are attractive materials for optoelectronics and in vivo biosensing. Researchers have now demonstrated that QDs, in addition to being excellent fluorescent probes, can be used as photoacoustic (which combines the advantages of optical absorption contrast with ultrasonic spatial resolution for deep imaging) and photothermal contrast agents and sensitizers, thereby providing an opportunity for multimodal high resolution photoacoustic/photothermal-fluorescent imaging as well as sensitizers in nanotherapeutic applications.
Nov 12th, 2008
 The study of individual live cells is a hugely important scientific task and essential to the field of molecular biology and biomedical research. Among the most significant technical challenges for performing successful live-cell imaging experiments is to maintain the cells in a healthy state and functioning normally on the microscope stage while being illuminated. Especially if scientists want to look into cellular processes that occur inside cells in their natural state and that cannot be observed by traditional cytological methods. Quantum dots (QDs), also called nanocrystals, hold increasing potential for in vitro and in vivo cellular imaging. For instance, we have previously reported about how researchers have used QDs for in vivo imaging of embryonic stem cells in mice, a novel technique that has opened up the possibility of using QDs for fast and accurate imaging applications in stem cell therapy. The usefulness of quantum dots comes from their peak emission frequency's extreme sensitivity to both the dot's size and composition. QDs have been touted as possible replacements for organic dyes in the imaging of biological systems, due to their excellent fluorescent properties, good chemical stability, broad excitation ranges and high photobleaching thresholds.
The study of individual live cells is a hugely important scientific task and essential to the field of molecular biology and biomedical research. Among the most significant technical challenges for performing successful live-cell imaging experiments is to maintain the cells in a healthy state and functioning normally on the microscope stage while being illuminated. Especially if scientists want to look into cellular processes that occur inside cells in their natural state and that cannot be observed by traditional cytological methods. Quantum dots (QDs), also called nanocrystals, hold increasing potential for in vitro and in vivo cellular imaging. For instance, we have previously reported about how researchers have used QDs for in vivo imaging of embryonic stem cells in mice, a novel technique that has opened up the possibility of using QDs for fast and accurate imaging applications in stem cell therapy. The usefulness of quantum dots comes from their peak emission frequency's extreme sensitivity to both the dot's size and composition. QDs have been touted as possible replacements for organic dyes in the imaging of biological systems, due to their excellent fluorescent properties, good chemical stability, broad excitation ranges and high photobleaching thresholds.
Oct 8th, 2008
 While the dye sensitized photovoltaic cell is a fairly mature design, researchers are still trying to improve its efficiency with various techniques, including structuring nanoporous electrodes to provide higher surface area and better charge transport, replacement of the liquid electrolyte by a solid one in order to prevent the electrolyte evaporation, and ways to widen the narrow absorption spectra of molecular dyes. In a standard DSSC, an organic molecule adsorbed on the surface of a porous electrode absorbs light and then initiates the charge separation process eventually leading to generation of photocurrent. One major difficulty in such cells is that very few dyes can absorb a broad spectral range, essentially covering the solar spectrum. In contrast, broad spectral coverage is an inherent property of semiconductor nanocrystals. The latter, however, turn out to do a rather lousy job in separating the charges. Researchers in Israel have now presented a new configuration for quantum dot sensitized DSSCs via a FRET process.
While the dye sensitized photovoltaic cell is a fairly mature design, researchers are still trying to improve its efficiency with various techniques, including structuring nanoporous electrodes to provide higher surface area and better charge transport, replacement of the liquid electrolyte by a solid one in order to prevent the electrolyte evaporation, and ways to widen the narrow absorption spectra of molecular dyes. In a standard DSSC, an organic molecule adsorbed on the surface of a porous electrode absorbs light and then initiates the charge separation process eventually leading to generation of photocurrent. One major difficulty in such cells is that very few dyes can absorb a broad spectral range, essentially covering the solar spectrum. In contrast, broad spectral coverage is an inherent property of semiconductor nanocrystals. The latter, however, turn out to do a rather lousy job in separating the charges. Researchers in Israel have now presented a new configuration for quantum dot sensitized DSSCs via a FRET process.
 Subscribe to our Nanotechnology Spotlight feed
Subscribe to our Nanotechnology Spotlight feed





