Showing Spotlights 433 - 440 of 559 in category All (newest first):
 Modern pharmaceutics is a very imprecise, wasteful and sometimes even dangerous discipline. Not only do most drugs fail even before they make it to market (about 80% of drugs never make it through clinical trials) but even the efficacy of many drugs that are being prescribed for certain diseases is questionable. The most important challenge, though, is to deliver the correct dose of a particular therapeutic (small molecules, proteins, or nuclei acids) to a specific disease site. Since this is generally unachievable, therapeutics have to be administered in excessively high doses, thereby increasing the odds of toxic side effects. Nanotechnology offers great visions of improved, personalized treatment of disease. The hope is that personalized medicine will make it possible to develop and administer for each individual patient the appropriate drug, at the appropriate dose, at the appropriate time. The benefits of this approach are accuracy, efficacy, safety and speed. Today, commercial nanomedicine is at a nascent stage of development and the full potential of nanomedicine is years or decades away. Currently the most advanced area of nanomedicine is the development and use of nanoparticles for drug delivery.
Modern pharmaceutics is a very imprecise, wasteful and sometimes even dangerous discipline. Not only do most drugs fail even before they make it to market (about 80% of drugs never make it through clinical trials) but even the efficacy of many drugs that are being prescribed for certain diseases is questionable. The most important challenge, though, is to deliver the correct dose of a particular therapeutic (small molecules, proteins, or nuclei acids) to a specific disease site. Since this is generally unachievable, therapeutics have to be administered in excessively high doses, thereby increasing the odds of toxic side effects. Nanotechnology offers great visions of improved, personalized treatment of disease. The hope is that personalized medicine will make it possible to develop and administer for each individual patient the appropriate drug, at the appropriate dose, at the appropriate time. The benefits of this approach are accuracy, efficacy, safety and speed. Today, commercial nanomedicine is at a nascent stage of development and the full potential of nanomedicine is years or decades away. Currently the most advanced area of nanomedicine is the development and use of nanoparticles for drug delivery.
Jul 25th, 2008
 Much is being written about nanotechnology's role in vastly improving the detection and treatment of cancer. Detection of cancer at the earliest stage provides the greatest chance of survival. Unfortunately, cancer has a logarithmic growth rate. A one cubic centimeter size tumor may have 40-50 cell divisions and typically doctors don't see 80% of the life of a tumor. The detection of a protein pattern in blood serum can be helpful in evidencing a possible presence of cancer at an early stage. The problem is that 'early' means the capability of detecting very few molecules in dilute conditions. Now, in another step to improve the design and fabrication of devices for single molecule detection, new research has demonstrated an experimental capability of detecting down to as few as 10 organic molecules deposited on a quantum dot.
Much is being written about nanotechnology's role in vastly improving the detection and treatment of cancer. Detection of cancer at the earliest stage provides the greatest chance of survival. Unfortunately, cancer has a logarithmic growth rate. A one cubic centimeter size tumor may have 40-50 cell divisions and typically doctors don't see 80% of the life of a tumor. The detection of a protein pattern in blood serum can be helpful in evidencing a possible presence of cancer at an early stage. The problem is that 'early' means the capability of detecting very few molecules in dilute conditions. Now, in another step to improve the design and fabrication of devices for single molecule detection, new research has demonstrated an experimental capability of detecting down to as few as 10 organic molecules deposited on a quantum dot.
Jul 23rd, 2008
 Modern medicine would be unthinkable without biomedical implants. The market for medical implant devices in the U.S. alone is estimated to be $23 billion per year and it is expected to grow by about 10% annually for the next few years. Implantable cardioverter defibrillators, cardiac resynchronization therapy devices, pacemakers, tissue and spinal orthopedic implants, hip replacements, phakic intraocular lenses and cosmetic implants will be among the top sellers. Current medical implants, such as orthopedic implants and heart valves, are made of titanium and stainless steel alloys, primarily because they are biocompatible. Unfortunately, a common complication associated with medical implants results from infectious biofilms which may cause chronic infection that is difficult to control. For instance, biofilms are present on the teeth as dental plaque, where they may become responsible for tooth decay and gum disease. Because teeth are easily accessible, removing plaque is not a problem. If biofilms develop on medical implants deep inside the body, though, they can become a serious, sometimes life-threatening problem. Preventing or limiting the formation of bacterial biofilm on the surface of implanted medical devices is an important approach to control bacterial biofilm-related infections. A new study demonstrates the effectiveness of a process combining surface nanocrystallization and thermal oxidation for reducing the biofilm's adherence to stainless steel.
Modern medicine would be unthinkable without biomedical implants. The market for medical implant devices in the U.S. alone is estimated to be $23 billion per year and it is expected to grow by about 10% annually for the next few years. Implantable cardioverter defibrillators, cardiac resynchronization therapy devices, pacemakers, tissue and spinal orthopedic implants, hip replacements, phakic intraocular lenses and cosmetic implants will be among the top sellers. Current medical implants, such as orthopedic implants and heart valves, are made of titanium and stainless steel alloys, primarily because they are biocompatible. Unfortunately, a common complication associated with medical implants results from infectious biofilms which may cause chronic infection that is difficult to control. For instance, biofilms are present on the teeth as dental plaque, where they may become responsible for tooth decay and gum disease. Because teeth are easily accessible, removing plaque is not a problem. If biofilms develop on medical implants deep inside the body, though, they can become a serious, sometimes life-threatening problem. Preventing or limiting the formation of bacterial biofilm on the surface of implanted medical devices is an important approach to control bacterial biofilm-related infections. A new study demonstrates the effectiveness of a process combining surface nanocrystallization and thermal oxidation for reducing the biofilm's adherence to stainless steel.
Jul 21st, 2008
 Any drug intended for systemic administration and all medical devices which will contact blood must undergo thorough biocompatibility testing. These tests include an in vitro assay to determine the material's potential to damage red blood cells (hemolysis). Hemolysis, the abnormal breakdown of red blood cells either in the blood vessels (intravascular hemolysis) or elsewhere in the body (extravascular), can lead to anemia or other pathological conditions. In the pharmaceutical industry, hematocompatibility testing is harmonized through the use of internationally recognized standard protocols. Nanotechnology- based medical devices and drug carriers are emerging as alternatives to conventional small-molecule drugs, and in vitro evaluation of their biocompatibility with blood components is a necessary part of early preclinical development. Many research papers have reported nanoparticle hemolytic properties but, so far, no in vitro hemolysis protocol has been available that is specific to nanoparticles. A new study published this month describes in vitro assays to study nanoparticle hemolytic properties, identifies nanoparticle interferences with these in vitro tests and provides the first comprehensive insight to potential sources of this interference, demonstrates the usefulness of including nanoparticle-only controls, and illustrates the importance of physicochemical characterization of nanoparticle formulations and visually monitoring test samples to avoid false-positive or false-negative results.
Any drug intended for systemic administration and all medical devices which will contact blood must undergo thorough biocompatibility testing. These tests include an in vitro assay to determine the material's potential to damage red blood cells (hemolysis). Hemolysis, the abnormal breakdown of red blood cells either in the blood vessels (intravascular hemolysis) or elsewhere in the body (extravascular), can lead to anemia or other pathological conditions. In the pharmaceutical industry, hematocompatibility testing is harmonized through the use of internationally recognized standard protocols. Nanotechnology- based medical devices and drug carriers are emerging as alternatives to conventional small-molecule drugs, and in vitro evaluation of their biocompatibility with blood components is a necessary part of early preclinical development. Many research papers have reported nanoparticle hemolytic properties but, so far, no in vitro hemolysis protocol has been available that is specific to nanoparticles. A new study published this month describes in vitro assays to study nanoparticle hemolytic properties, identifies nanoparticle interferences with these in vitro tests and provides the first comprehensive insight to potential sources of this interference, demonstrates the usefulness of including nanoparticle-only controls, and illustrates the importance of physicochemical characterization of nanoparticle formulations and visually monitoring test samples to avoid false-positive or false-negative results.
Jul 18th, 2008
 Scientists consider the exploration of carbon nanotubes (CNTs) for biomedical applications as a field with significant potential, leading to applications where CNTs could act as magnetic nano-heaters, drug-carrier systems and sensors which allow a diagnostic and therapeutic usage on a cellular level. The EU, for instance, has funded a four-year nanotechnology research program that studies the chemical and physical properties of CNTs in order to find mechanisms which can be applied for a biomedical purpose in appropriate medical devices. Studies of their interaction with biological environments (immune response, toxicity, interaction with the single cell) will provide the basis for applying the CNT for imaging (nanoparticles-based contrast agents), sensoring (nanoparticles-based diagnostics) and cancer treatment (hyperthermia, nanotechnology-based targeted drug delivery). A new study that exploits the unique properties of single-walled CNTs (SWCNT) for biomedical purposes shows the use of SWCNTs as an efficient platform for immunotherapeutic applications. Scientists demonstrate the surface area tunability of SWCNT bundles by chemical treatment and its effect on antibody adsorption and subsequent T cell activation. T cells are central players in initiating and maintaining immune responses. An important goal of successful immunotherapy is the stimulation of T cell immune responses against targets of interest such as tumors.
Scientists consider the exploration of carbon nanotubes (CNTs) for biomedical applications as a field with significant potential, leading to applications where CNTs could act as magnetic nano-heaters, drug-carrier systems and sensors which allow a diagnostic and therapeutic usage on a cellular level. The EU, for instance, has funded a four-year nanotechnology research program that studies the chemical and physical properties of CNTs in order to find mechanisms which can be applied for a biomedical purpose in appropriate medical devices. Studies of their interaction with biological environments (immune response, toxicity, interaction with the single cell) will provide the basis for applying the CNT for imaging (nanoparticles-based contrast agents), sensoring (nanoparticles-based diagnostics) and cancer treatment (hyperthermia, nanotechnology-based targeted drug delivery). A new study that exploits the unique properties of single-walled CNTs (SWCNT) for biomedical purposes shows the use of SWCNTs as an efficient platform for immunotherapeutic applications. Scientists demonstrate the surface area tunability of SWCNT bundles by chemical treatment and its effect on antibody adsorption and subsequent T cell activation. T cells are central players in initiating and maintaining immune responses. An important goal of successful immunotherapy is the stimulation of T cell immune responses against targets of interest such as tumors.
Jul 10th, 2008
 Cells are the basic building blocks of life. The ability to sense and modify intracellular processes is important for, among other things, bettering our understanding of biological processes, developing drugs and evaluating their effectiveness, and modifying cell function. Due to the cell's small size and fragility, probing the cell's interior with high precision is not a simple task. To address this challenge, researchers have developed nanoscale, carbon-based cellular probes ('carbon nanopipettes' or CNP). The CNP consists of a glass capillary lined with a carbon film along its inner surface and terminating with an exposed carbon nanopipe. The probes are fabricated through a process that does not require any assembly and that facilitates quantity fabrication. Depending on controllable process conditions, the carbon tip's diameter may vary from tens to hundreds of nanometers and its length can range from zero to a few micrometers.
Cells are the basic building blocks of life. The ability to sense and modify intracellular processes is important for, among other things, bettering our understanding of biological processes, developing drugs and evaluating their effectiveness, and modifying cell function. Due to the cell's small size and fragility, probing the cell's interior with high precision is not a simple task. To address this challenge, researchers have developed nanoscale, carbon-based cellular probes ('carbon nanopipettes' or CNP). The CNP consists of a glass capillary lined with a carbon film along its inner surface and terminating with an exposed carbon nanopipe. The probes are fabricated through a process that does not require any assembly and that facilitates quantity fabrication. Depending on controllable process conditions, the carbon tip's diameter may vary from tens to hundreds of nanometers and its length can range from zero to a few micrometers.
Jul 8th, 2008
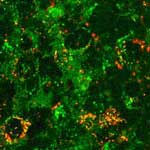 The challenge in treating most brain disorders is overcoming the difficulty of delivering therapeutic agents to specific regions of the brain by crossing the blood-brain barrier (BBB). This barrier - a tight seal of endothelial cells that lines the blood vessels in the brain - is a physiological checkpoint that selectively allows the entry of certain molecules from blood circulation into the brain. The problem for scientists is that the BBB does not differentiate what it keeps out. BBB strictly limits transport into the brain through both physical (tight junctions) and metabolic (enzymes) barriers. With very few exceptions, only nonionic and low molecular weight molecules soluble in fat clear the BBB. For instance, alcohol, caffeine, nicotine and antidepressants meet these criteria. However, large molecules needed to deliver drugs do not. The utter scarcity of techniques for brain-specific delivery of therapeutic molecules using non-invasive approaches (like drilling a hole in the skull) has led researchers to increasingly explore the vast potential of nanotechnology toward the diagnosis and treatment of diseases/disorders incurable with present techniques.
The challenge in treating most brain disorders is overcoming the difficulty of delivering therapeutic agents to specific regions of the brain by crossing the blood-brain barrier (BBB). This barrier - a tight seal of endothelial cells that lines the blood vessels in the brain - is a physiological checkpoint that selectively allows the entry of certain molecules from blood circulation into the brain. The problem for scientists is that the BBB does not differentiate what it keeps out. BBB strictly limits transport into the brain through both physical (tight junctions) and metabolic (enzymes) barriers. With very few exceptions, only nonionic and low molecular weight molecules soluble in fat clear the BBB. For instance, alcohol, caffeine, nicotine and antidepressants meet these criteria. However, large molecules needed to deliver drugs do not. The utter scarcity of techniques for brain-specific delivery of therapeutic molecules using non-invasive approaches (like drilling a hole in the skull) has led researchers to increasingly explore the vast potential of nanotechnology toward the diagnosis and treatment of diseases/disorders incurable with present techniques.
Jul 3rd, 2008
 Elastomeric (i.e. elastic) proteins are able to withstand significant deformations without rupture before returning to their original state when the stress is removed. Consequently, these proteins confer excellent mechanical properties to many biological tissues and biomaterials. Depending on the role performed by the tissue or biomaterial, elastomeric proteins can behave either as springs or shock absorbers. Recent scientific work in Canada resulted in the engineering of the first artificial chameleon elastomeric proteins that mimic and combine these two different behaviors into one protein. Under the regulation of a molecular regulator, these designer proteins exhibit one of the two distinct mechanical behaviors - spring or shock absorber - which closely mimic the two extreme behaviors observed in naturally occurring elastomeric proteins.
Elastomeric (i.e. elastic) proteins are able to withstand significant deformations without rupture before returning to their original state when the stress is removed. Consequently, these proteins confer excellent mechanical properties to many biological tissues and biomaterials. Depending on the role performed by the tissue or biomaterial, elastomeric proteins can behave either as springs or shock absorbers. Recent scientific work in Canada resulted in the engineering of the first artificial chameleon elastomeric proteins that mimic and combine these two different behaviors into one protein. Under the regulation of a molecular regulator, these designer proteins exhibit one of the two distinct mechanical behaviors - spring or shock absorber - which closely mimic the two extreme behaviors observed in naturally occurring elastomeric proteins.
Jul 2nd, 2008
 Modern pharmaceutics is a very imprecise, wasteful and sometimes even dangerous discipline. Not only do most drugs fail even before they make it to market (about 80% of drugs never make it through clinical trials) but even the efficacy of many drugs that are being prescribed for certain diseases is questionable. The most important challenge, though, is to deliver the correct dose of a particular therapeutic (small molecules, proteins, or nuclei acids) to a specific disease site. Since this is generally unachievable, therapeutics have to be administered in excessively high doses, thereby increasing the odds of toxic side effects. Nanotechnology offers great visions of improved, personalized treatment of disease. The hope is that personalized medicine will make it possible to develop and administer for each individual patient the appropriate drug, at the appropriate dose, at the appropriate time. The benefits of this approach are accuracy, efficacy, safety and speed. Today, commercial nanomedicine is at a nascent stage of development and the full potential of nanomedicine is years or decades away. Currently the most advanced area of nanomedicine is the development and use of nanoparticles for drug delivery.
Modern pharmaceutics is a very imprecise, wasteful and sometimes even dangerous discipline. Not only do most drugs fail even before they make it to market (about 80% of drugs never make it through clinical trials) but even the efficacy of many drugs that are being prescribed for certain diseases is questionable. The most important challenge, though, is to deliver the correct dose of a particular therapeutic (small molecules, proteins, or nuclei acids) to a specific disease site. Since this is generally unachievable, therapeutics have to be administered in excessively high doses, thereby increasing the odds of toxic side effects. Nanotechnology offers great visions of improved, personalized treatment of disease. The hope is that personalized medicine will make it possible to develop and administer for each individual patient the appropriate drug, at the appropriate dose, at the appropriate time. The benefits of this approach are accuracy, efficacy, safety and speed. Today, commercial nanomedicine is at a nascent stage of development and the full potential of nanomedicine is years or decades away. Currently the most advanced area of nanomedicine is the development and use of nanoparticles for drug delivery.
 Subscribe to our Nanotechnology Spotlight feed
Subscribe to our Nanotechnology Spotlight feed





