Showing Spotlights 473 - 480 of 625 in category All (newest first):
 Lithium-ion batteries seem to be everywhere these days. They power most of the electronic devices we carry around with us - cell phones, laptops, MP3 players, digital cameras and so on. They get their name from the lithium ion that moves from the anode to the cathode during discharge and from the cathode to the anode during recharging. Due to their good energy-to-weight ratios, lithium batteries are some of the most energetic rechargeable batteries available today. In terms of weight and size, batteries have become one of the limiting factors in the continuous process of developing smaller and higher performance electronic devices. To meet the demand for batteries having higher energy density and improved cycle characteristics, researchers have been making tremendous efforts to develop new electrode materials or design new structures of electrode materials. Demonstrating the benefits of directed nanostructure-design of electrode materials, Chinese scientists have prepared tin nanoparticles encapsulated in elastic hollow carbon spheres. This tin-based nanocomposite exhibits a very high specific capacity, excellent cycling performance, and therefore shows great potential as anode materials in lithium-ion batteries.
Lithium-ion batteries seem to be everywhere these days. They power most of the electronic devices we carry around with us - cell phones, laptops, MP3 players, digital cameras and so on. They get their name from the lithium ion that moves from the anode to the cathode during discharge and from the cathode to the anode during recharging. Due to their good energy-to-weight ratios, lithium batteries are some of the most energetic rechargeable batteries available today. In terms of weight and size, batteries have become one of the limiting factors in the continuous process of developing smaller and higher performance electronic devices. To meet the demand for batteries having higher energy density and improved cycle characteristics, researchers have been making tremendous efforts to develop new electrode materials or design new structures of electrode materials. Demonstrating the benefits of directed nanostructure-design of electrode materials, Chinese scientists have prepared tin nanoparticles encapsulated in elastic hollow carbon spheres. This tin-based nanocomposite exhibits a very high specific capacity, excellent cycling performance, and therefore shows great potential as anode materials in lithium-ion batteries.
Apr 7th, 2008
 You probably have seen quite a number of research reports on the amazing climbing abilities of geckos. Here at Nanowerk, we ran several Spotlights on this topic, for instance on mimicking gecko toe structures to fabricate super-strong dry adhesives. One demonstration of so-called 'gecko tape' has already been used in building Stickybot, a quadruped robot capable of climbing smooth vertical surfaces, such as glass, acrylic and whiteboard. In addition to the animal kingdom, scientists have started looking at plants to identify biological climbing mechanisms that could be exploited for engineering applications. One obvious candidate is ivy, a climbing woody plant. Researchers now have found that ivy secretes nanoparticles which allow the plant to affix to a surface and play an important role in the plant's climbing capability. This ivy secretion mechanism may inspire new, 'green' methods for synthesizing nanoparticles biologically or new approaches to adhesion mechanisms for mechanical devices.
You probably have seen quite a number of research reports on the amazing climbing abilities of geckos. Here at Nanowerk, we ran several Spotlights on this topic, for instance on mimicking gecko toe structures to fabricate super-strong dry adhesives. One demonstration of so-called 'gecko tape' has already been used in building Stickybot, a quadruped robot capable of climbing smooth vertical surfaces, such as glass, acrylic and whiteboard. In addition to the animal kingdom, scientists have started looking at plants to identify biological climbing mechanisms that could be exploited for engineering applications. One obvious candidate is ivy, a climbing woody plant. Researchers now have found that ivy secretes nanoparticles which allow the plant to affix to a surface and play an important role in the plant's climbing capability. This ivy secretion mechanism may inspire new, 'green' methods for synthesizing nanoparticles biologically or new approaches to adhesion mechanisms for mechanical devices.
Mar 31st, 2008
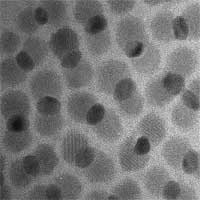 The past few years have seen tremendous progress in developing and fine-tuning fabrication methods for nanoparticles. An important research direction in nanoparticle synthesis is the expansion from single-component nanoparticles to hybrid nanostructures that possess two or more functional properties thanks to the integration of different materials. Multifunctional nanocarriers are a particularly hot topic in nanomedicine where it is hoped that such particles can significantly enhance the efficacy of many therapeutic and diagnostic protocols. What makes hybrid multicomponent nanostructures so alluring is not only the combination of different functionalities, but also the possibility to independently optimize the dimension and material parameters of the individual components. Apart from their multifunctionality, another advantage of these structures is that they can provide novel functions not available in single-component materials. A recent feature article provides an overview of the synthetic efforts of multicomponent hybrid nanoparticles via high-temperature solution-phase synthesis. The topics include chemical synthesis of multicomponent nanoparticles; characterization of the structural and physical properties, especially the ones arising from the interactions between different components; and potential applications of these multicomponent hybrid nanoparticles.
The past few years have seen tremendous progress in developing and fine-tuning fabrication methods for nanoparticles. An important research direction in nanoparticle synthesis is the expansion from single-component nanoparticles to hybrid nanostructures that possess two or more functional properties thanks to the integration of different materials. Multifunctional nanocarriers are a particularly hot topic in nanomedicine where it is hoped that such particles can significantly enhance the efficacy of many therapeutic and diagnostic protocols. What makes hybrid multicomponent nanostructures so alluring is not only the combination of different functionalities, but also the possibility to independently optimize the dimension and material parameters of the individual components. Apart from their multifunctionality, another advantage of these structures is that they can provide novel functions not available in single-component materials. A recent feature article provides an overview of the synthetic efforts of multicomponent hybrid nanoparticles via high-temperature solution-phase synthesis. The topics include chemical synthesis of multicomponent nanoparticles; characterization of the structural and physical properties, especially the ones arising from the interactions between different components; and potential applications of these multicomponent hybrid nanoparticles.
Mar 19th, 2008
 Understanding and manipulating cellular function at the level of individual molecules is within reach. One of the requirements of single molecule techniques is the ability to follow an individual molecule for sufficiently long times in solution. However, it is a challenge to cope with the effects of Brownian motion (the random motion of small particles suspended in a gas or liquid) on this time scale. To meet this challenge, more recently, biomolecules have been encapsulated inside lipid vesicles, which are themselves tethered to a surface. Now, a novel nanocontainer offers controlled permeability functionality which not only is desirable for single molecule imaging but also is a very important property for micro- and nanodevices and for delivery of drugs or imaging agents in vitro and in vivo.
Understanding and manipulating cellular function at the level of individual molecules is within reach. One of the requirements of single molecule techniques is the ability to follow an individual molecule for sufficiently long times in solution. However, it is a challenge to cope with the effects of Brownian motion (the random motion of small particles suspended in a gas or liquid) on this time scale. To meet this challenge, more recently, biomolecules have been encapsulated inside lipid vesicles, which are themselves tethered to a surface. Now, a novel nanocontainer offers controlled permeability functionality which not only is desirable for single molecule imaging but also is a very important property for micro- and nanodevices and for delivery of drugs or imaging agents in vitro and in vivo.
Mar 18th, 2008
 You have seen the effect: if you splash water on your car it leaves wet areas; if you do this with your freshly polished car the drops just pearl off. Materials scientists are very interested in designing surfaces that allow them to control this effect - called wetting - because it enables them to fabricate things like more comfortable contact lenses, better prosthetics, and self-cleaning materials. The primary measurement to determine wettability is the angle between the solid surface and the surface of a liquid droplet on the solid's surface. For example, a droplet of water on a hydrophobic surface would have a high contact angle, but a liquid spread out on a hydrophilic surface would have a small one. Maintaining the position of a drop of water on a hydrophobic surface (e.g. your newly waxed car) appears to be impossible - it will just move across the surface. Scientists in Israel have managed to fabricate a nanostructured, highly hydrophobic surface that allows them to pin a nearly spherical drop of water in place. A droplet sitting on one class of these substrates did not fall even after the substrate was turned upside-down! An important application for this novel fabrication technique could be as a tool in single-molecule spectroscopy: a water drop, containing molecules to be probed, could be pinned down for an extended time, allowing to spectroscopically probe it for long periods without affecting the properties of molecules, or even just one molecule, dissolved in the water drop.
You have seen the effect: if you splash water on your car it leaves wet areas; if you do this with your freshly polished car the drops just pearl off. Materials scientists are very interested in designing surfaces that allow them to control this effect - called wetting - because it enables them to fabricate things like more comfortable contact lenses, better prosthetics, and self-cleaning materials. The primary measurement to determine wettability is the angle between the solid surface and the surface of a liquid droplet on the solid's surface. For example, a droplet of water on a hydrophobic surface would have a high contact angle, but a liquid spread out on a hydrophilic surface would have a small one. Maintaining the position of a drop of water on a hydrophobic surface (e.g. your newly waxed car) appears to be impossible - it will just move across the surface. Scientists in Israel have managed to fabricate a nanostructured, highly hydrophobic surface that allows them to pin a nearly spherical drop of water in place. A droplet sitting on one class of these substrates did not fall even after the substrate was turned upside-down! An important application for this novel fabrication technique could be as a tool in single-molecule spectroscopy: a water drop, containing molecules to be probed, could be pinned down for an extended time, allowing to spectroscopically probe it for long periods without affecting the properties of molecules, or even just one molecule, dissolved in the water drop.
Mar 13th, 2008
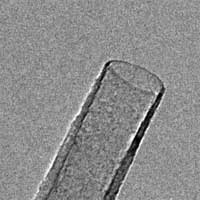 'Field evaporation' is the phenomenon by which surface atoms are ionized (evaporated) under an applied, extremely high electric field of the order of several volts per nanometer. Electric fields of this magnitude can only be achieved by applying a high field to an extremely sharp needle such as the specimen tip in a Field Ion Microscope. Field evaporation was first reported over 50 years ago and has since developed into the powerful Atom Probe Field Ion Microscopy which is able to reproduce the atomic structure of a piece of material in three dimensions. Today, field evaporation is mainly used for material characterization, and the behavior of nanomaterials at extremely strong electric fields is of great scientific and technological interest. In principle, the field evaporation phenomenon can be utilized not only for materials characterization, but also for materials processing and morphology control with extremely high precision because of its unique atom-by-atom removal capability. However, detailed structural evolution of nanomaterials during field evaporation has never been directly observed and this limitation has greatly restricted the potential applications of field evaporation as a materials-processing tool. Now, researchers in Beijing have reported the first direct observation of field evaporation phenomena using a transmission electron microscopy (TEM) technique. By conducting in situ TEM field evaporation experiments on individual carbon nanotubes (CNTs), the researchers were able to reveal details about the structural evolution of the nanomaterials via direct observation. Using this technique, they have been able to perform controlled engineering of the CNTs with atomic precision, for example, grinding and shortening of CNTs, shaping of the open ends of CNTs, and opening of CNT caps.
'Field evaporation' is the phenomenon by which surface atoms are ionized (evaporated) under an applied, extremely high electric field of the order of several volts per nanometer. Electric fields of this magnitude can only be achieved by applying a high field to an extremely sharp needle such as the specimen tip in a Field Ion Microscope. Field evaporation was first reported over 50 years ago and has since developed into the powerful Atom Probe Field Ion Microscopy which is able to reproduce the atomic structure of a piece of material in three dimensions. Today, field evaporation is mainly used for material characterization, and the behavior of nanomaterials at extremely strong electric fields is of great scientific and technological interest. In principle, the field evaporation phenomenon can be utilized not only for materials characterization, but also for materials processing and morphology control with extremely high precision because of its unique atom-by-atom removal capability. However, detailed structural evolution of nanomaterials during field evaporation has never been directly observed and this limitation has greatly restricted the potential applications of field evaporation as a materials-processing tool. Now, researchers in Beijing have reported the first direct observation of field evaporation phenomena using a transmission electron microscopy (TEM) technique. By conducting in situ TEM field evaporation experiments on individual carbon nanotubes (CNTs), the researchers were able to reveal details about the structural evolution of the nanomaterials via direct observation. Using this technique, they have been able to perform controlled engineering of the CNTs with atomic precision, for example, grinding and shortening of CNTs, shaping of the open ends of CNTs, and opening of CNT caps.
Mar 7th, 2008
 When two surfaces approach each other in air, they attract. A phenomenon that is explained by van der Waals forces that affect molecules' interaction. Without these - very weak - intermolecular forces, life as we know it would be impossible. They are responsible for a number of properties of molecular compounds, including crystal structures, condensing, melting and boiling points, surface tension, and densities. Intermolecular forces form molecules like enzymes, proteins, and DNA into the shapes required for biological activity. Van der Waals forces can also be repulsive, for instance when two surfaces approach each other in liquid: the same force which causes attraction in air (and which is responsible for so called stiction and adhesion) can be made repulsive by choosing the right combination of surface materials and intervening liquid. This force has the characteristic that it increases very rapidly with very small changes in separation when the surfaces are close to each other. Researchers now have shown that if repulsive van der Waals forces exist between two surfaces prior to their contact then friction is essentially precluded and supersliding is achieved. This opens the possibility that, in certain material systems, the controlled use of repulsive van der Waals forces could be a way to reduce, if not eliminate, friction.
When two surfaces approach each other in air, they attract. A phenomenon that is explained by van der Waals forces that affect molecules' interaction. Without these - very weak - intermolecular forces, life as we know it would be impossible. They are responsible for a number of properties of molecular compounds, including crystal structures, condensing, melting and boiling points, surface tension, and densities. Intermolecular forces form molecules like enzymes, proteins, and DNA into the shapes required for biological activity. Van der Waals forces can also be repulsive, for instance when two surfaces approach each other in liquid: the same force which causes attraction in air (and which is responsible for so called stiction and adhesion) can be made repulsive by choosing the right combination of surface materials and intervening liquid. This force has the characteristic that it increases very rapidly with very small changes in separation when the surfaces are close to each other. Researchers now have shown that if repulsive van der Waals forces exist between two surfaces prior to their contact then friction is essentially precluded and supersliding is achieved. This opens the possibility that, in certain material systems, the controlled use of repulsive van der Waals forces could be a way to reduce, if not eliminate, friction.
Feb 28th, 2008
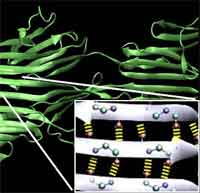 Much has been written about the fascinating properties of spider silk, a biopolymer that is stronger than steel and more elastic than rubber. The silken threads possess a unique combination of mechanical properties: strength, extensibility and toughness. Of course this begs the obvious question: How is it possible that spider silk, produced by little creatures that evolved about 400 million years ago, can be as strong as steel - a modern alloy that plays a critical role in our infrastructure and which still attracts considerable R&D investments in its production technology? What is perplexing is that the atomic interactions (H-bonds) in spider silk are actually 100 to 1,000 times weaker than those in steel, or than those in the superfiber Kevlar, where covalent bonds are used. Hydrogen bonds are the basic chemical bonds that hold together proteins, similar to trusses and beams in buildings, and play a key role in controlling the behavior of these structures. In order to reach silk's mechanical properties, most synthetic materials must be much denser, thus much heavier and consume much more energy during their synthesis and transport. New analysis performed at MIT's Laboratory for Atomistic and Molecular Mechanics shows that the intriguing strength of spider silk may be made possible by precisely controlling the number and the geometry of H-bonds at a characteristic length scale. The physical concept is that by making many small elements work together cooperatively, the weaknesses of the individual components can be overcome. All this must happen at the nanoscale in order to be effective.
Much has been written about the fascinating properties of spider silk, a biopolymer that is stronger than steel and more elastic than rubber. The silken threads possess a unique combination of mechanical properties: strength, extensibility and toughness. Of course this begs the obvious question: How is it possible that spider silk, produced by little creatures that evolved about 400 million years ago, can be as strong as steel - a modern alloy that plays a critical role in our infrastructure and which still attracts considerable R&D investments in its production technology? What is perplexing is that the atomic interactions (H-bonds) in spider silk are actually 100 to 1,000 times weaker than those in steel, or than those in the superfiber Kevlar, where covalent bonds are used. Hydrogen bonds are the basic chemical bonds that hold together proteins, similar to trusses and beams in buildings, and play a key role in controlling the behavior of these structures. In order to reach silk's mechanical properties, most synthetic materials must be much denser, thus much heavier and consume much more energy during their synthesis and transport. New analysis performed at MIT's Laboratory for Atomistic and Molecular Mechanics shows that the intriguing strength of spider silk may be made possible by precisely controlling the number and the geometry of H-bonds at a characteristic length scale. The physical concept is that by making many small elements work together cooperatively, the weaknesses of the individual components can be overcome. All this must happen at the nanoscale in order to be effective.
Feb 14th, 2008
 Lithium-ion batteries seem to be everywhere these days. They power most of the electronic devices we carry around with us - cell phones, laptops, MP3 players, digital cameras and so on. They get their name from the lithium ion that moves from the anode to the cathode during discharge and from the cathode to the anode during recharging. Due to their good energy-to-weight ratios, lithium batteries are some of the most energetic rechargeable batteries available today. In terms of weight and size, batteries have become one of the limiting factors in the continuous process of developing smaller and higher performance electronic devices. To meet the demand for batteries having higher energy density and improved cycle characteristics, researchers have been making tremendous efforts to develop new electrode materials or design new structures of electrode materials. Demonstrating the benefits of directed nanostructure-design of electrode materials, Chinese scientists have prepared tin nanoparticles encapsulated in elastic hollow carbon spheres. This tin-based nanocomposite exhibits a very high specific capacity, excellent cycling performance, and therefore shows great potential as anode materials in lithium-ion batteries.
Lithium-ion batteries seem to be everywhere these days. They power most of the electronic devices we carry around with us - cell phones, laptops, MP3 players, digital cameras and so on. They get their name from the lithium ion that moves from the anode to the cathode during discharge and from the cathode to the anode during recharging. Due to their good energy-to-weight ratios, lithium batteries are some of the most energetic rechargeable batteries available today. In terms of weight and size, batteries have become one of the limiting factors in the continuous process of developing smaller and higher performance electronic devices. To meet the demand for batteries having higher energy density and improved cycle characteristics, researchers have been making tremendous efforts to develop new electrode materials or design new structures of electrode materials. Demonstrating the benefits of directed nanostructure-design of electrode materials, Chinese scientists have prepared tin nanoparticles encapsulated in elastic hollow carbon spheres. This tin-based nanocomposite exhibits a very high specific capacity, excellent cycling performance, and therefore shows great potential as anode materials in lithium-ion batteries.
 Subscribe to our Nanotechnology Spotlight feed
Subscribe to our Nanotechnology Spotlight feed





