| Posted: Nov 30, 2009 | |
Nanotechnology trap captures cancer cells in blood |
|
| (Nanowerk Spotlight) Metastasis – the most common cause of cancer-related death in patients with solid tumors – is caused by marauding tumor cells that break off from the primary tumor site and ride in the bloodstream to set up colonies in other parts of the body. These breakaway cancer cells in the peripheral blood are known as circulating tumor cells (CTCs). Detecting and analyzing these cells can provide critical information for managing the spread of cancer and monitoring the effectiveness of therapies. | |
| Whereas the analysis of metastatic solid biopsy samples is still the best way of examining a tumor, this technique is difficult or impossible to apply in the early stages of cancer. By capturing CTCs, doctors can essentially perform a liquid biopsy by capturing break-away cells floating in the bloodstream. Isolating CTCs with techniques involving involve immunomagnetic beads or microfluidic devices has been technically challenging due to the extremely low abundance (a few to hundreds per milliliter) of CTCs among a large number (hundreds of millions per milliliter) of hematologic cells in the blood. | |
| Nanotechnology researchers have now developed a an efficient cell-capture platform based on 3D nanostructured substrates. The device is engineered out of nanoscale silicon pillars and has managed to capture up to 65 percent of circulating tumor cells in lab samples within human blood – far more than any existing diagnosis tool for CTC capture. | |
| "Our nanopillar chip captured more than 10 times the amount of cells captured by the currently used flat structure," says Shutao Wang, a postdoctoral researcher in Hsian-Rong Tseng's research group at the David Geffen School of Medicine at UCLA and the California NanoSystems Institute at UCLA. | |
| Wang explains that the uniqueness of this new approach lies in the use of 3D nanostructured substrates – specifically, a silicon-nanopillar array – which allow for enhanced local topographic interactions between the nanopillar substrates and nanoscale components of the cellular surface and result in vastly improved cell-capture affinity compared to unstructured, i.e., flat, silicon substrates. | |
| The team has reported their findings in a recent online issue of Angewandte Chemie International ("Three-Dimensional Nanostructured Substrates toward Efficient Capture of Circulating Tumor Cells"). | |
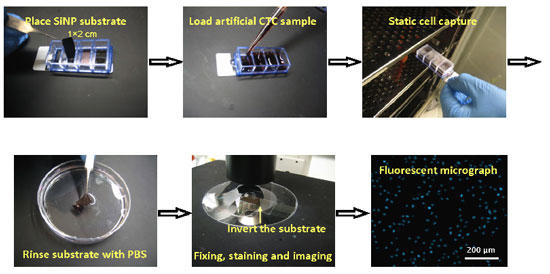 |
|
| The photos show the simple and convenient procedures of cell capture experiments using the 3D nanostructured substrates. (Reprinted with permission from Wiley-VCH Verlag) | |
| Lithographic tools for creating chips for computers were employed to prepare the cell capturing device. Using a wet chemical etching method, the team began by fabricating a 1-by-2-centimeter silicon chip with densely packed nanopillars with diameters of 100–200 nm and lengths varying from 1 to 20 µm. The nanopillars were then coated with streptavidin. Finally, biotinylated anti-EpCAM (epithelial-cell adhesion molecule) – an antibody protein that can help recognize and capture tumor cells – was introduced onto the streptavidin-coated substrates prior to the cell-capture experiments. | |
| To test cell-capture performance, Wang and his team incubated the nanopillar chip in a culture medium with breast cancer cells. As a control, they performed a parallel experiment with a cell-capture method that uses a chip with a flat surface. They found that their nanopillar device captured 45 to 65 percent of the cancer cells in the medium, compared with only 4 to 14 percent for the flat device. | |
| The nanopillar chip uses a common chamber slide, which fits into standard laboratory cell incubators. After the chip has been incubated and immunofluorescence-stained, an automated fluorescence microscope is used to identify and count the CTCs. The very simple device setting on the chamber slide allows multiple CTC detections to occur at the same time. | |
| As they tried to optimize their device, the researchers noticed that the number of substrate-immobilized cells increases with increasing incubation time. To determine the minimum time required to achieve cell capture, they examined the cell-capture performances at different incubation times and with various cell types. They found that for the EpCAM-positive cells, the maximal cell-capture numbers were achieved at an incubation time of 45 minutes. This compares to the upwards of three to four hours it takes for CTC detection using CellSearch, a technology currently approved by the U.S. Food and Drug Administration. This makes it conceivable that this novel nanotechnology platform can provide a convenient and cost-efficient alternative for CTC sorting in clinics. | |
| "The impact of our cell-capture platform beyond CTCs will potentially benefit the diagnosis of early diseases that are currently detected by means of cell-capture technologies," says Wang. "Importantly, cell viability with this platform is as high as 84–91%, which is conducive to subsequently releasing the cells, culturing them, and performing molecular biological diagnosis." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
