| Posted: May 21, 2007 | |
Protein printboards |
|
| (Nanowerk Spotlight) In 2005, researchers in the Netherlands developed the concept of a "molecular printboard" (named for its parallels with a computer motherboard) - a monolayer of host molecules on a solid substrate on which guest molecules can be attached with control over position, binding strength, and binding dynamics. Molecules can be positioned on the printboard using supramolecular contact printing and supramolecular dip-pen nanolithography. In this way, nanoscale patterns can be written and erased on the printboard. This technique, which combines top-down fabrication (lithography) with bottom-up methods (self-assembly), has now been applied to proteins. The resulting "protein printboards", allowing the capture and immobilization of proteins with precise control over specificity, strength and orientation, allows the fabrication of protein chips for applications in proteomics. They will play a major role in unraveling the human protein map, just as special chips were instrumental in mapping human DNA. | |
| The attachment of proteins to surfaces is a key step in many biotechnological processes and applications. They usually require complete control over the adsorption strength and reversibility, protein orientation, and retention of biological function – requirements that can only be met when the binding of the protein to the surface is specific. | |
| "Proteins don't let themselves be caught as easily as DNA" Dr. Jurriaan Huskens tells Nanowerk. "A molecular printboard with receptors for proteins is not sufficient for this task because proteins undergo non-specific interactions too easily; although the protein can be bound to the board it does not retain its shape and function. To prevent this non-specific binding, our research group put a new type of linker on the board. These small molecules make the surface 'invisible' for proteins and thus the proteins can be attached 'without knowing it'." | |
| Huskens is Professor of Supramolecular Chemistry & Nanofabrication in the Molecular Nanofabrication Group (MnF) and MESA+ Program Director of Nanofabrication at the University of Twente in the Netherlands. Together with researchers from the Biozentrum at the University of Frankfurt in Germany, Huskens and his group developed the use of β-cyclodextrin (βCD) molecular printboards as a general platform for the immobilization of proteins through small multivalent, orthogonal linker molecules. | |
| In this technique, the proteins bind to connecting molecules, so-called linkers, which, in turn connect to the surface of the board. The linker molecule, hexa (ethylene glycol) mono (adamantyl ether), forms a dynamic second layer on top of the cyclodextrin, which can prevent unwanted effects like non-specific binding of protein, but can make way for the 'right' proteins to be bound to the surface in the desired orientation. | |
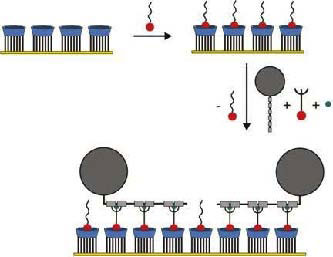 |
Nonspecific protein adsorption is prevented by masking the surface with small molecules. Only proteins that can be connected to special linker molecules will be attached to the surface. (Image: MESA+ Institute for Nanotechnology) |
| "βCD is a well known host for various small hydrophobic organic molecules in aqueous environments" says Huskens. "We have modified βCD with seven heptathioether chains to obtain ordered and densely packed self-assembled monolayers on gold. The use of these multivalent host–guest interactions allows the formation of kinetically stable assemblies, and thus local complex formation by patterning, for example, so that these surfaces can be viewed as 'molecular printboards'." | |
| So far, non-specific interactions for protein-protein interactions had to be suppressed with special measures. Thanks to the small linker molecules used, this process now has been simplified . The researchers have proven that the method is suitable, for instance, for attaching the frequently used strapavidin-biotin-couple, and for proteins that are functionalized with a so-called histidine tag (histidine is one of the 20 most common natural amino acids present in proteins). | |
| The latter is developed in cooperation with the group of Robert Tampé in Frankfurt, and is of utmost importance, since many proteins can be functionalized with such a histidine tag. | |
| The researchers are further developing this method for the attachment of antibodies and cells to protein-functionalized surfaces. | |
| "Our implementation of this supramolecular nonspecific protein inhibition scheme demonstrates the strong potential for the use of molecular printboards as a general platform for the immobilization of proteins" says Huskens. "We will now try to develop models for describing the multivalent thermodynamics of such orthogonal systems, and to increase the complexity of the protein architectures to antibodies and cells." | |
| The results of this work were reported in a paper, titled "Molecular Printboards as a General Platform for Protein Immobilization: A Supramolecular Solution to Nonspecific Adsorption" in the April 19, 2007 online edition of Angewandte Chemie. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com.
