| Posted: May 09, 2011 | |
Desert beetle shows researchers how to capture spilled oil underwater |
|
| (Nanowerk Spotlight) In the wake of the BP oil spill in the Gulf of Mexico we published a Nanowerk Spotlight on Nanotechnology-based solutions for oil spills that provided a general overview of the wide variety of nanomaterials and nanotechnologies that offer significant promise for oil spill cleanup and recovery. One problem with many existing solutions though is that they are one-offs, i.e. one they absorb oil they can't be re-used and need to be disposed of (which could in turn create secondary pollution effects). | |
| Ideally, any oil absorbent material used during ocean oil spills should be reusable and with special wettability that could controllably capture and release oil pollution repeatedly. Addressing this issue, researchers have now created an underwater water/solid interface inspired by fish scales. The surface of this new material shows superamphiphobicity in air and superoleophilicity under water, allowing it to be repeatedly used to capture and collect oil droplets in water. | |
| Reporting their findings in the May 2, 2011 online edition of Advanced Materials ("Underwater Oil Capture by a Three-Dimensional Network Architectured Organosilane Surface"), a research team from Dalian Maritime University and the Beijing National Laboratory for Molecular Sciences, have developed a novel organosilane surface which mimics the 'desert beetle effect' (this beetle relies on its bumpy back, consisting of alternating wax-coated hydrophobic regions and non-waxy hydrophilic regions, to capture drinking water from fog-laden wind) underwater and could repeatedly capture and collect oil droplets in water. | |
| "We synthesized the organosilane surfaces by a simple phase separation reaction and grafted to glass substrates," Meihua Jin, a researcher at the Department of Materials Science and Engineering at Dalian Maritime University, and first author of the paper, explains to Nanowerk. "Hydrolysis of the Si-Cl organosilane monomer yielded Si-OH and polycondensation formed highly cross-linked 3D networks." | |
| Scanning electron microscopy shows that the surface is composed of nanofibers and microbumps, which criss-cross each other and form a 3D network architecture. | |
| "It is well known that the wettability of a surface is governed by the surface free-energy as well as the surface roughness," says Jin. "Our organosilane-grafted surface is of a relatively low free-energy and high roughness, its wettability is thereby evaluated by a contact angle system. in air, the contact angle of water and oil are 168.2 ± 1.3° and 148.1 ± 2.1°, respectively. This result confirms that the surface presents a typical superamphiphobic property in air. The rough structure formed by the 3D nanofibers and microbumps contributes greatly to an increase in the amphiphobicity, and makes the surface amphihydrophobic." | |
 |
|
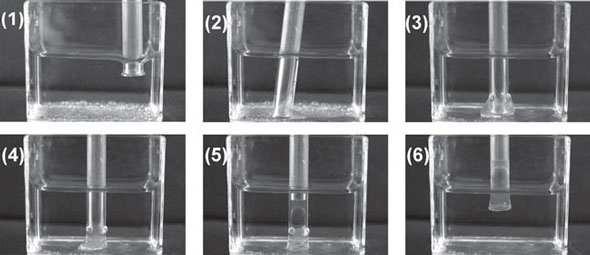
| The oil capture and collection process with a organosilane surface glass tube. Step 1: a layer of oil droplets is sprayed in bottom of a water container. Step 2: Glass tube touches and captures the oil drops underwater. Step 3: as the glass tube moves, oil drops are gathered together. Step 4–6: the oil droplets are sucked off from the water. (Reprinted with permission from Wiley-VCH Verlag) | |
| To demonstrate that their novel material can be used to collect oil droplets in water, the team carried out an experiment to determine the capability to capture oil on its surface (see images above): A layer of oil droplets was sprayed in a container filled with water. As the glass tube was moved underwater, the oil droplets gathered at the bottom of the glass tube coalesced together and could then be easily extracted from the water. | |
| "After the organosilane surface was removed from the water surface, the superamphiphobic surface in air was easily cleaned by flushing," says Jin. "In other words, the oil droplet collected underwater could fall off from the surface automatically and quickly." | |
| This study opens up a new strategy to the disposal of oily wastewater, which is certainly significant for future industrial applications. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
