| Sep 18, 2018 | |
Nanotechnology based drug delivery systems for nanomedicine |
|
| (Nanowerk Spotlight) Development of an effective approach for delivering a new drug is as important as inventing a new drug. Even if a developed new drug has excellent pharmacokinetic and therapeutic properties, it shows its potential activity in the body effectively only when it is exactly targeted to specific molecules. | |
| Various nanotechnological approaches for effective drug delivery have been developed and some of them have already been successfully commercialized. Most prominent nano drug delivery systems that are in market place are oncology related and based on liposomal, solid nanoparticle based, protein polymer conjugates and polymer-drug conjugate based delivery platforms. | |
Bioavailability – the problem with conventional drug delivery |
|
| The bioavailability of a drug within the body depends on several factors like the size of the drug molecules and solubility parameters. Conventional dosage forms therefore face challenges in reaching the target site at appropriate dose. | |
| For example, conventional dosage forms of some of the highly water soluble drugs cause fluctuations in drug concentration in the body due to high disintegration properties and also result in faster clearance of the drug from the blood stream. | |
| Other drugs are fat soluble and when taken in conventional dosage forms may cause bioavailability problems. Similarly, patients suffering from chronic diseases like diabetes need to take painful insulin injections on a regular basis. | |
| Also, cancer patients regularly have to undergo powerful chemotherapy, which involves quite severe side effects as the anticancer drugs target cancer cells and normal cells equally. | |
| Hence proper platforms to deliver the drugs at targeted sites without losing their efficacies while limiting the associated side effects are highly required. | |
| Many novel technologies for developing effective drug delivery systems came into existence among which nanotechnology platforms for achieving targeted drug delivery are gaining prominence these days. Research in this field includes the development of drug nanoparticles, polymeric and inorganic biodegradable nanocarriers for drug delivery, and surface engineering of carrier molecules. | |
| These nanocarriers help in solubilizing the lipophilic drugs, protecting fragile drugs from enzymatic degradation, pH conditions, etc., and targeting specific sites with triggered release of drug contents. | |
Nanobots |
|
| Nanobots or nanomotors are advanced sub-micron sized, self driven, biodegradable nanodevices made of bio-nano components, which carry cargo to the target sites. | |
| This active motor based drug delivery approach promises an effective and improved drug delivery compared to conventional methods. Gold nanoparticle loaded PEDOT/zinc-based artificial micromotors are tested in mouse models via oral administration. They showed excellent acid-driven, self propulsive properties with high cargo-loading capacities. | |
| Unimolecular submersible nanomachines that are activated by UV light, DNA-origami based nanorobots, light-induced actuating nanotransducers, WiNoBots, magnetic multilink nanoswimmers, etc., are some of the other technological developments that are anticipating the application of nanorobots in drug delivery. | |
Nanoghosts |
|
| Nanoghost technology is one of the latest approaches developed for smart drug/gene delivery. Nanoghosts are a type of nanovesicles derived from naturally functionalized mammalian cell surface membranes of whole biological cells such as mesenchymal stem cells (MSCs) that are devoid of cytoplasm and organelles. These naturally derived carriers overcome drug loading issues, evade tumor specific immune responses, provide greater nanoparticle stability and improve drug release profiles. | |
Nanoclews |
|
| Nanoclew or nanococoon is a DNA based biocompatible drug delivery system. In this system single stranded DNA makes whole nanococoon. It self assembles to look like a yarn or cocoon or a clew like structure by rolling-circle amplification. | |
| For instance, scientists have used DNA nanoclews to shuttle CRISPR-Cas9 gene-editing tool into cells. | |
| Biomedical engineering researchers also developed a drug delivery system consisting of nanococcons made of DNA that target cancer cells and trick the cells into absorbing the cocoon before unleashing anticancer drugs. | |
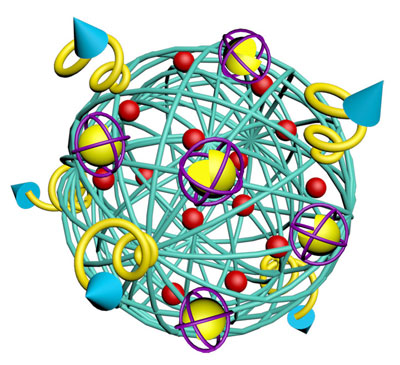 |
|
| The nano-cocoon has ligands on its surface that bind to receptors on the surface of cancer cells. (Image: Zhen Gu, University of North Carolina at Chapel Hill) | |
Nanoneedles |
|
| Direct delivery of therapeutic molecules to cell cytoplasm is highly desirable in drug delivery for achieving high efficacy and reduced side effects. But biological membranes act as strong barriers for entry of drug into the cell. | |
| Nanoneedles could help to overcome these problems. Nanoneedles are very small and make temporary perforations to biological membranes. Hence these needles, without disturbing biological functions of the body can deliver the drugs. | |
Nanoclusters |
|
| Metal nanoclusters are self assembled nanoparticles made of polymers or small organic molecules crosslinked with plasmonic metals (such as gold, silver, or magnetic particles). Because of their molecular-like and fluorescence properties, they have gained importance in the field of drug delivery as well as biosensing and bioimaging. | |
Nanobubbles |
|
| Nanobubbles are gas-filled spherical nano-sized structures often stabilized by polymeric/lipid shells. These nanocarriers in combination with thermal, ultrasound, acoustic or magnetic sensitivities are used as more efficient imaging and drug delivery agents in various therapeutic treatments. They are more stable and showed longer residence time in systemic circulation. | |
| For instance researchers have shown that dynamically tuned intracellular plasmonic nanobubbles are well suited for cell theranostics since they combine diagnosis (through optical scattering), therapy (through mechanical, nonthermal and selective damage of target cells) and optical guidance of the therapy into one fast process. | |
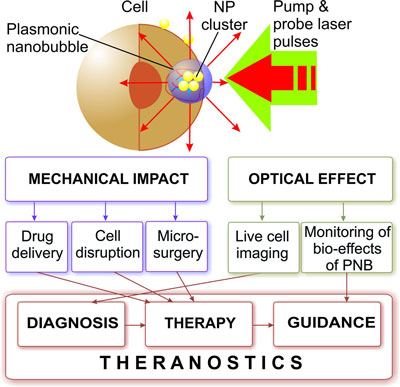 |
|
| Mechanism and medical applications of plasmonic nanobubbles. (Illustration: Dr. Lapotko, Rice University) | |
Exosomes |
|
| Exosomes – tiny biological nanoparticles which transfer information between cells – offer significant potential in detecting and treating disease, | |
| Exosomes are the most promising next generation ?natural? nanovehicles for targeted drug and gene delivery. These are nano-sized vesicles (with diameter 40-200 nm) derived from patients? own healthy cells with an exceptional ability to interact with cellular membranes. | |
| They possess a unique property of 'cell specific tropism' (target specific cells by displaying receptors in the membranes) towards the originated cells, which can be utilized as a delivery strategy to transport cargo consisting of drugs, proteins and microRNAs. | |
| Since they are of biological origin containing natural lipid bilayers, immunogenicity and drug clearance from the body can be reduced and can easily cross the blood-brain barrier, which are beneficial for designing personalized therapeutic approaches. | |
Injectable Nanoparticle Generator (iNG) |
|
| Injectable Nanoparticle Generator is the first of its kind new drug delivery strategy developed by the scientists at Houston Methodist Research Institute in Texas. It consists of a Dox loaded polymer made up of multiple strands enwrapped in a biodegradable nanoporous silicon material. | |
| When injected intravenously, due to natural tropism, they accumulate at the tumors, where the silicon material degrades slowly releasing the drug polymeric strands. These strands spontaneously form nanoparticles that are then taken up by the cancer cells. | |
| The acidic environment inside the cancer cells triggers the polymeric strands to release the drug. This kind of novel approach helps the drug to cross multiple biological barriers in order to achieve targeted therapy. | |
Nano-terminators |
|
| Researchers at North Carolina State University developed biodegradable liquid metal nano-terminators that are drug loaded nanodroplets made of a liquid-phase eutectic gallium-indium core and a thiolated polymeric shell and thiolated hyaluronic acid) to target cancer cells. | |
| These droplets when injected into the blood stream get absorbed into the tumors and release the drug by dissolving the liquid metal due to the presence of highly acidic tumour environment. | |
Dendrimers |
|
| Dendrimers are nanopolymers with a well defined structure, which is different from linear polymer molecules. It has a core at the center consisting of an atom or a molecule and branches emerging from core comprises of repeated units having one branch junction, called as generations, and many terminal groups also at the surface of the generations. Hence the framework of dendrimer can be controlled to make a good carrier. | |
| Read more in our article on dendrimer nanomedicine – developing efficient therapeutic strategies for the treatment of neurological disorders. | |
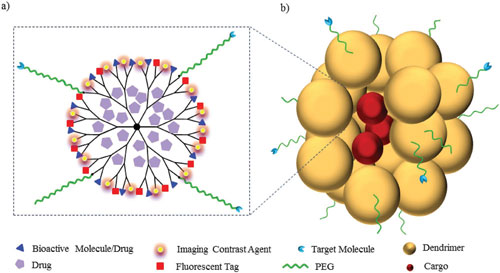 |
|
| a) Dendrimer multifunctionalization and ?dendritic box?. b) Dendritic nanoparticle (cargo = drug, nucleic acid, protein, peptide, among others). (© Wiley-VCH Verlag) (click on image to enlarge) | |
Liposomes |
|
| Liposomes are spherical vesicles composed of an aqueous core surrounded by a lipid bilayer. Liposomes have been used to improve bioavailability and absorption of drug and to reduce toxicity. | |
| The distinctive feature of liposomes is its ability to compartmentalize and solubilize both hydrophilic and hydrophobic materials. For several years now, significant research has been underway on liposomal delivery of different drugs. | |
| One such example is ATP-mediated liposomal drug delivery. The method can be likened to keeping a cancer-killing bomb and its detonator separate until they are inside a cancer cell, where they then combine to destroy the cell. | |
Niosomes |
|
| Niosomes are also known as nonionic surfactant vesicles with sizes ranging from 20 nm to around 50 µm. They are formed from self-assembly of hydrated synthetic nonionic surfactant monomers and are capable of entrapping a variety of drugs. Niosomes have been evaluated as an alternative to liposomes in order to overcome their stability problems. Their unique structure helps to encapsulate both hydrophilic and lipophilic drug substances. Entrapment efficiency increases with increase in the concentration and lipophilicity of the surfactant used. | |
| In cancer therapy active targeting involves recognition of specific site (targeted site) and delivery to that place. It was achieved by coupling drug or ligand with the delivery system, which can interact with specific receptor at the targeted site and can be released from the carrier to commence destructive action on cell. | |
Carbon nanotubes (CNTs) |
|
| Carbon nanotubes offer a number of advantages for delivering drugs to specific locations inside the body which suggest that they may provide an improved result over nanoparticles. They have a larger inner volume which allows more drug molecules to be encapsulated, and this volume is more easily accessible because the end caps can be easily removed, and they have distinct inner and outer surfaces for functionalization. | |
| Carbon nanotubes can also be chemically modified to carry a variety of molecules such as drugs, DNA, proteins, peptides, targeting ligands etc. into cells – which makes them suitable candidates for targeted delivery applications. | |
| Nitrogen-doped carbon nanotubes for instance have been developed for drug delivery applications. | |
| Scientists have developed a cancer immunotherapy that rapidly grows and enhances a patient?s immune cells outside the body using carbon nanotube / polymer composites; the immune cells can then be injected back into a patient?s blood to boost the immune response or fight cancer. | |
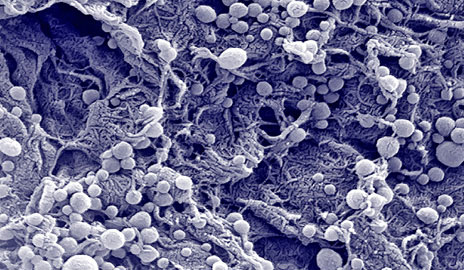 |
|
| A high-resolution, scanning electron microscope image of the carbon nanotube-polymer composite. The bundled CNTs appear as spaghetti-like structures. (Image: Yale University) | |
| However, the issue of cytotoxicity of CNTs is an area that has already attracted much research interest and has not resulted in a definitive answer yet. Given the inconclusive state of these nanotoxicology studies researchers says that more systematic biological evaluations of CNTs having various chemical and physical properties are warranted in order to determine their precise pharmacokinetics, cytotoxicity, and optimal dosages. | |
Graphene |
|
| Another novel carbon nonmaterial, graphene is beginning to be researched for nanomedicine applications as well. | |
| For instance, researchers have developed a simple method to thermally ablate highly resistant cancer cells using targeted biodegradable graphene nanoparticles. | |
| Scientists also have used graphene to target and neutralise cancer stem cells while not harming other cells. This new development opens up the possibility of preventing or treating a broad range of cancers, using a non-toxic material. | |
| Other novel applications involve graphene-based sensors to detect cancer cells. | |
Conclusion |
|
| Nanotechnologies have enabled novel solutions for the treatment of various diseases. Nanodrug delivery systems present some advantages than conventional drug delivery systems such as high cellular uptake and reduced side effects. Development of drug delivery systems by using nanotechnology particularly for cancer treatment is making revolutionary changes in treatment methods and handling side effects of chemotherapy. | |
| Furthermore, nanotechnology allows for selective targeting of disease and infection containing cells and malfunctioned cells. | |
| Nanotechnology has also opened new opportunities in implantable delivery systems such as use in bone cement, nanoneedle patches, etc which are preferable than using other modes of administration like injections and oral delivery. | |
| Liposomes and dendrimers are already in the market for decades, but other nanotechnology based drug delivery systems such as nanobots, nanoclusters, nanoghosts and nanoclews are currently at research and development stage that requires rigorous clinical trials, to prove their efficacy. | |
| Although the technology has great potential, cost factor becomes a hindrance in its use. Moreover novel nanoscale systems lack long-term safety data which need to be addressed. | |
| Apart from these challenges nanomaterials and their approval methods are not well defined till now, which brings another limitation and takes even more time in developing clinically useful nanotechnology based drug delivery systems. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
