| Aug 21, 2019 | |
Pre-coating liposomes with an artificial protein corona reduces capture by immune system |
|
| (Nanowerk Spotlight) Despite considerable, decade-long efforts in the field of nanomedicine, fewer nanoparticle technologies than expected have made it to clinical trials. As pointed out in a recent opinion piece in Trends in Biotechnology ("Debugging Nano–Bio Interfaces: Systematic Strategies to Accelerate Clinical Translation of Nanotechnologies"), "the wide gap between the efforts and effective clinical translation is, at least in part, due to multiple overlooked factors in both in vitro and in vivo environments; a poor understanding of the nano–bio interface; and misinterpretation of the data collected in vitro – all of which reduce the accuracy of predictions regarding the nanoparticles' fate and safety in humans." | |
| When nanoparticles enter the blood stream or other biological fluids, they are quickly surrounded by a layer of proteins. This so-called protein corona on the nanoparticles hinders interactions between the targeting ligands on the nanoparticles and their binding partners on the cells' surface (read more: "Personalized protein coronas result in different therapeutic or toxic impacts of identical nanoparticles"). | |
| Understanding the role of this protein corona appears to be a critical factor for successful clinical translation of medical nanotechnologies. | |
| New research results published in Nature Communications ("Interplay of protein corona and immune cells controls blood residency of liposomes") demonstrate that the protein corona controls the interaction of liposomes with immune cells. | |
| In this work, the researchers investigated the interplay between positive, negative, and neutral liposomes with peripheral blood mononuclear cells in the presence of protein corona. | |
 |
|
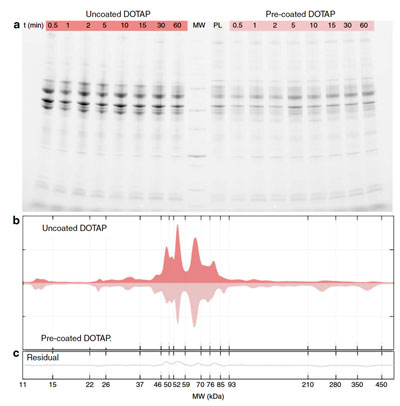
| Protein corona of uncoated and pre-coated liposomes in patients’ plasma. a SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis) gel of human plasma proteins obtained from uncoated and pre-coated DOTAP liposomes following incubation in patients’ plasma at different time points. The molecular weights of the proteins in the standard ladder (MW) and the protein pattern of pre-coated liposomes (i.e., before incubation in patients’ plasma; labelled as PL) are reported in the middle for reference. b One-dimensional (1D) averaged protein profiles of coronas formed around uncoated and pre-coated DOTAP after exposure to patients’ plasma. c Residual obtained by subtracting the 1D profile of pre-coated from that of uncoated DOTAP. (© Nature Communications) (click on image to enlarge) | |
| The information about the interplay between nanoparticles and the immune system can help make accurate predictions of their blood residency time and therefore nanoparticles' therapeutic efficacy. | |
| "We also reveal that the pre-coating of liposomes with an artificial corona made of human plasma proteins significantly decreases capture by circulating leukocytes in whole blood, thus, representing an efficient strategy to escape sequestration by immune cells and enable prolonged circulation in vivo," Morteza Mahmoudi, Assistant Professor, Precision Health Program at Michigan State University, and one of the authors of the paper, tells Nanowerk. | |
| Liposomes are one of the most versatile colloidal carrier systems for drug and gene delivery since they have structural features that allow the encapsulation, protection, and transportation of molecules with different physical–chemical properties. | |
| "Despite the ease of preparation and functionalization of liposomes, their clinical application is associated with numerous drawbacks," Mahmoudi explains. "More than three decades of drug-delivery research have correlated opsonization – the process by which a pathogen is marked for ingestion and eliminated by the phagocytes – by human plasma proteins with uptake of liposomes by resident macrophages and fast removal from bloodstream. However, previous studies demonstrated that protein corona can recruit specific proteins from plasma, which eventually prevent particle recognition by macrophages." | |
| A major aspect of the work reported in the paper focuses on the question, whether the pre-adsorbed corona proteins may allow liposomes to avoid capture by circulating leukocytes when they are exposed to patient's whole blood. | |
| According to the researchers, this question needs to be addressed in order to reveal if liposomes pre-coating may be an effective strategy to enable prolonged circulation in vivo. | |
| "Plasma protein concentration is a major factor affecting biological identity of nanoparticles," Mahmoudi points out. "Therefore, mechanistic understanding of liposomes’ biological identity as a function of protein concentration is a necessary step to engineer pre-coated liposomes with proper physico-chemical properties – e.g., size and surface charge – and protein corona composition." | |
| The team's results suggest that cationic liposomes pre-coated with artificial protein corona at low protein concentration could be ideal nanocarriers for targeting macrophages in cancer. This opens up the potential to exploit protein corona formation, which will significantly influence the development of novel nanomaterials. | |
| As an additional important conclusion, these results suggest that in vitro investigations performed at low plasma concentration may better mimic the behavior of liposomes in vivo thus contributing to predict immune response. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
