| Posted: Nov 06, 2008 | |
Multifunctional nanoparticles do double duty in fight against cancer |
|
| (Nanowerk Spotlight) Mesoporous materials, i.e. materials with pores that measure less than 50 nanometers in size, have been researched extensively for at least 20 years now. Especially mesoporous silicates, due to their large surface area, their uniform pore size, and the accessibility of these pores, have become very popular as catalyst materials and excellent dug delivery candidates. | |
| Despite of the long research history of mesoporous silica materials and their biomedical potential, there have been only few reports on actual in vivo applications. Although the theory looks good, there are several practical obstacles for mesoporous silica materials to be used as drug carriers or for instance as in vivo cancer targeting agents. Researchers who tried making small mesoporous silica particles with sizes of around 100 nm often ended up with much larger lumps of aggregated particles. These larger chunks cannot be used because, due to their size, they are easily trapped in the body's defense mechanism, the reticuloendothelial system (RES). | |
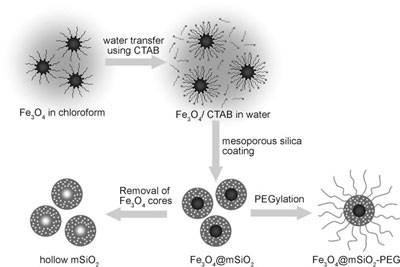
| Researchers in South Korea have now reported the fabrication of discrete, monodisperse, and precisely size-controllable core–shell nanoparticles that are smaller than 100 nm, by using single magnetite nanocrystals as core and a mesoporous silica shell. Thanks to this combination, these nanoparticles provide multifunctional capabilities – they are magnetic, fluorescent, and porous (to transport drug molecules, DNA or proteins) at the same time. This makes them a novel and very attractive candidate for future cancer diagnosis and therapy. | |
| "The magnetite core of the nanoparticles that we synthesized, and whose size can be easily controlled below 100nm, allows them to be used as T2 MRI contrast agent and the dye-doped silica shells as fluorescent imaging agent" Dr. Taeghwan Hyeon explains to Nanowerk. "In addition, anticancer small molecule drugs can be incorporated in 3 nm-sized pores in the mesoporous silica shell. We injected core-shell nanoparticles via the tail vein of a tumor-bearing mouse and found that these multifunctional nanoparticles can be accumulated at tumor cells via passive targeting. The tumor can be detected through MRI and the fluorescence from sectioned tumor tissue confirmed the accumulation of core-shell nanoparticles in tumor sites." | |
| Professor Hyeon is Director, National Creative Research Initiative, Center for Oxide Nanocrystalline Materials at Seoul National University. Together with members of his Center and colleagues from Diagnostic Radiology, Seoul National University Hospital and the Institute of Radiation Medicine, Medical Research Center at Seoul National University, he presents the team's novel nanoparticles in a recent issue of Angewandte Chemie International Edition ("Multifunctional Uniform Nanoparticles Composed of a Magnetite Nanocrystal Core and a Mesoporous Silica Shell for Magnetic Resonance and Fluorescence Imaging and for Drug Delivery"). | |
 |
|
| Schematic illustration of the synthetic procedure for magnetite nanocrystal/mesoporous silica core–shell nanoparticles. (Reprinted with permission from Wiley) | |
| Hyeon's group is well-known for the large-scale synthesis of uniform-sized nanoparticles. In their highly cited 2004 paper in Nature Materials for instance, they describe the synthesis of as much as 40 grams of monodisperse nanocrystals in a single reaction, without a size-sorting process. Moreover, the particle size could be controlled simply by varying the experimental conditions ("Ultra-large-scale syntheses of monodisperse nanocrystals"). | |
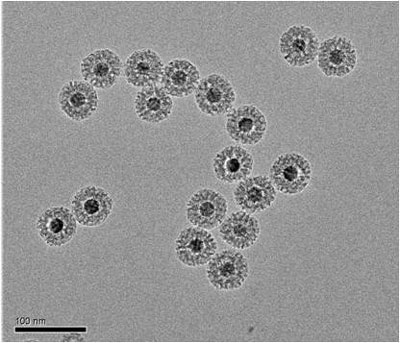
| The Korean team describes their novel core-shell nanoparticles as being discrete without aggregation and well-dispersed in aqueous media. In addition they can easily control the particle size from 50 nm to 100 nm by simple changing the ratio of magnetite seed and silica precursors. | |
| "To increase the biocompatibility of the nanoparticles in vivo, we functionalized the surface of the core-shell nanoparticles with poly(ethylene glycol) (PEG), a well-known biocompatible polymer" says Hyeon. "The PEG-coated nanoparticles are stable in dispersion and the hydrodynamic size of the resulting core-shell nanoparticles is around 100 nm. This is smaller than the vascular leakage in tumor sites to pass into the tumor but lager than normal vascular pores." | |
| An interesting aspect of this work is that the mesoporous silica shell can be coated on other nanocrystals with different compositions and shapes. | |
| "For example," says Hyeon "we successfully synthesized manganese oxide/silica core-shell particles using 25 nm manganese oxide nanocrystals that have recently been used as a T1 MRI contrast agent. We were also able to coat mesoporous silica uniformly on one-dimensional nanostructures such as nanotubes using a very similar synthetic procedure to that employed for the spherical nanocrystals. Since most uniform and highly crystalline nanocrystals are synthesized by the thermal decomposition method and are therefore hydrophobic, our simple and highly reproducible synthetic process can be a good protocol for the generalized fabrication of uniform nanocrystal core /mesoporous silica shell structures." | |
| In their experiments, the scientists found that 2 hours after the injection into mice, the accumulation of the nanoparticles in tumors could be detected in magnetic resonance images. Even at 24 hours after injection the nanoparticles still remained in tumor sites. | |
 |
|
| TEM images of core–shell magnetite/mesoporous silica nanoparticles. (Image: Dr. Hyeon/Seoul National University) | |
| According to Hyeon, the Korean team believes that this is the first report on the accumulation of intravenously injected mesoporous silica nanoparticles in a tumor site. "Our particles are stable under physiological conditions and circulate for a long enough time to accumulate in the tumor sites through the enhanced permeability and retention effect. In terms of their in vivo biocompatibility, we observed no distinct toxicity in the nude mice administered with the particles over the monitoring period." | |
| In order to further improve the cancer targeting performance of these nanoparticles, the team is now trying to modify the surface of the particles with different densities of PEG and different biocompatible polymers, and to conjugate cancer targeting antibody or peptide. | |
| They also want to spend more efforts on exactly figuring out the potential cytotoxic effect of these materials on healthy cells. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com.
