| Posted: Oct 04, 2011 | |
New nanomaterials for radioactive waste clean-up in water |
|
| (Nanowerk Spotlight) Back in 2008 we reported on nanotechnology solution for radioactive waste cleanup, specifically the use of titanate nanofibers as absorbents for the removal of radioactive ions from water. Now, the same group that developed these nanomaterials reports in a new study that the unique structural properties of titanate nanotubes and nanofibers make them superior materials for removal of radioactive cesium and iodine ions in water. | |
| Radioactive cesium and iodine ions are products of uranium fission and can be easily dissolved in water during an accident at a nuclear reactor like the one in Fukushima earlier this year. The fear is that these fission products could get into the groundwater and could make their way into the food chain. | |
| As we reported in our previous Nanowerk Spotlight mentioned above, natural inorganic cation exchange materials, such as clays and zeolites, have been extensively studied and used in the removal of radioactive ions from water via ion exchange and are subsequently disposed of in a safe way. However, synthetic inorganic cation exchange materials – such as synthetic micas, g-zirconium phosphate, niobate molecular sieves, and titanate – have been found to be far superior to natural materials in terms of selectivity for the removal of radioactive cations from water. Radioactive cations are preferentially exchanged with sodium ions or protons in the synthetic material. More importantly, a structural collapse of the exchange materials occurs after the ion exchange proceeds to a certain extent, thereby forming a stable solid with the radioactive cations being permanently trapped inside. Hence, the immobilized radioactive cations can be disposed safely. | |
| "Based on our earlier work, we have now demonstrated a potentially cost-effective method to remediate cesium and iodine ions from contaminated water by using the unique chemistry of titanate nanotubes and nanofibers to chemisorb these ions," HuaiYong Zhu, a professor of chemistry at the Queensland University of Technology, tells Nanowerk. | |
| The team, which reported their findings in the September 20, 2011 online edition of Angewandte Chemie International Edition ("Capture of Radioactive Cesium and Iodide Ions from Water by Using Titanate Nanofibers and Nanotubes"), also found that the new sorbents can not only take up these ions but efficiently trap them for safe disposal because of their unique structural and chemical features. | |
 |
|
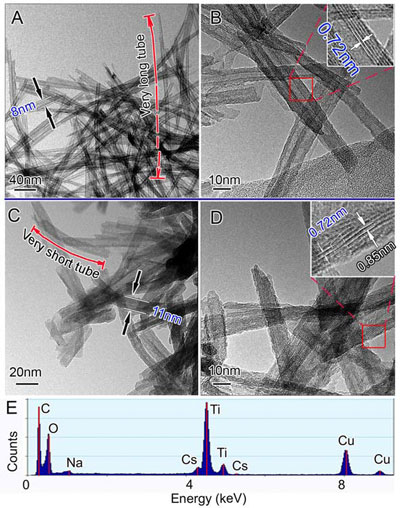
| PTEM micrographs of the tubular sorbents before and after entrapment of cesium ions. Panel A and B are the TEM images of the initial T3NT. Inset in panel B is a HRTEM image of the selected area in panel B. Panel C and D are the TEM images of the nanotubes after adsorption of cesium ions (Cs-T3NT). Inset in panel D is a HRTEM image of the selected area in panel D, in which tube walls with two different interlayer distances are connected by a trapezoid neck. Panel E is the EDS analysis of Cs-T3NT. (Reprinted with permission from Wiley-VCH Verlag) | |
| "The sorbents take up cesium ions via an exchange with sodium ions in the nanostructures; the rapid uptake of cesium ions eventually triggers a phase transition of the titanate and traps the cesium ions inside permanently for safe disposal," explains Zhu. "This is because the fibers and tubes consist of negatively charged thin layers – as thin as two oxygen atoms – and phase transition of the layers with low rigidity can be readily induced." | |
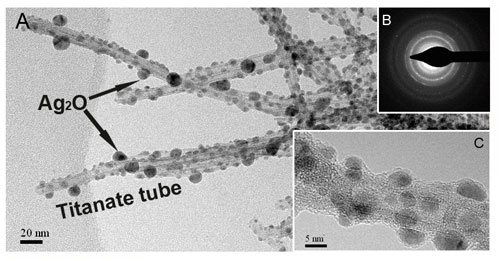
| In order to capture and immobilize iodine ions from water, the researchers anchored silver oxide nanocrystals on the external surfaces of titanate nanotubes and nanofibers by chemical bonds owing to their crystallographic similarity. These composites can efficiently capture iodine ions forming silver iodine precipitate on the titanates. | |
 |
|
| Silver oxide nanocrystals anchored sodium titanate nanotubes (Ag2O-NT). (A) Typical TEM image depicting the abundant silver oxide nanocrystals (∼5-10 nm) coated on titanate nanotubes. (B)The selected area Electron Diffraction Pattern (EDP) of the nanotubes. (C) HRTEM image of a single nanotube and the anchored silver oxide nanocrystals. (Reprinted with permission from Wiley-VCH Verlag) | |
| Zhu explains that this work introduces three new concepts: | |
| 1) Utilizing ion exchange to uptake cesium ions; which eventually triggers a phase transition of the titanate trapping the ions inside permanently for safe disposal. | |
| 2) To attach silver oxide nanocrystals to the external surface of the nanostructures via coherent interface between the crystal and the substrate, for silver oxide nanocrystals to capture iodine ions efficiently. It is impractical to use fine silver oxide powder directly for the removal of iodine anions because the separation of the used silver oxide particles from water will be extremely difficult and costly, and aggregation of the fine nanocrystals also substantially reduces the removal efficiency. | |
| 3) The new adsorbents can be readily dispersed in liquid and easily separated after purification for safe disposal due to their one-dimensional morphology; and the adsorption beds loaded with the adsorbents can permit high flux. This significantly enhances the adsorption efficiency and reduces the separation costs. Furthermore, the titanate nanofibers and nanotubes can be easily synthesized from titanium compounds including titanium dioxide at low cost. | |
| Given that there are hundreds of nuclear power stations over the world, and hundreds more in planning, developing efficient adsorbents is of great significance for the nuclear industry, not to mention our environment. Even if all these nuclear power plants will be shut down eventually, the necessary clean-up work will still have to be done. | |
| "The adsorbent of titanate nanofibers and nanotubes not only can be produced from titanium dioxide at low cost, but the ability to tailor these structural features to enhance uptake and trapping of ions can be exploited for further development of new and selective adsorbents for the removal of other toxic cations and anions that may be found in groundwater or wastewater," says Zhu. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
