| Posted: Jun 06, 2014 | |
An integrated solar-powered energy conversion-storage-utilization system |
|
| (Nanowerk Spotlight) Almost all strategies for solar energy harvest and solar energy storage that exist today are developed as independent technologies. For instance, a solar cell generates electricity from the absorption and conversion of sunlight, while the storage of the produced electricity has to be implemented with another set of energy utilization solutions such as batteries/supercapacitors and fuel cells. | |
| Photoelectrochemical (PEC) conversion based on semiconductor materials is a highly important and promising approach for utilizing solar energy with minimal carbon emissions. Today, it is one of the most sustainable methods of producing hydrogen – when sun hits the PEC cell, the solar energy is absorbed and used for splitting water molecules into its components, hydrogen and oxygen. | |
| One of the largest challenges facing PEC water splitting technologies – and other solar conversion techniques as well – is the selection and design of semiconductor photoelectrode materials/structures, due to multiple stringent requirements including photoelectrochemical stable, appropriate band gap size and band edge position, fast charge transfer and low recombination rates, and efficient hydrogen/oxygen evolution. | |
| In addition, the waste of the oxidative energy in producing oxygen from water splitting, and the loss of electric energy when delivering electron flows into an external energy storage device, are two additional important factors that limit the efficient utilization of the solar energy. | |
| With quite an ingenious solution, researchers have now demonstrated a hybrid, multifunctional material system that allows for simultaneous solar power generation (respectively hydrogen production), electrical energy storage, and chemical sensing. | |
| "The development of our work offers opportunities for direct converting the solar energy into two different forms of energy that can be directly utilized, i.e., hydrogen gas at the photocathode and supercapacitive energy at the photoanode," Gengfeng Zheng, a professor of chemistry at Fudan University, tells Nanowerk. "Our system obtains a high pseudocapacitance of up to 455 F g-1 with repeating charging-discharging capability. More importantly, the NiO nanomaterials grown on the photoanode can further serve as an excellent glucose sensor using the stored electrochemical energy, with a high sensitivity up to 0.1 µM." | |
| As the team reports in the May 13, 2014 online edition of Nano Letters ("Fully Solar-Powered Photoelectrochemical Conversion for Simultaneous Energy Storage and Chemical Sensing"), this means that the fully solar-powered energy storage and utilization device also serves as a glucose sensor by directly utilizing solar energy for in situ glucose detection. | |
 |
|
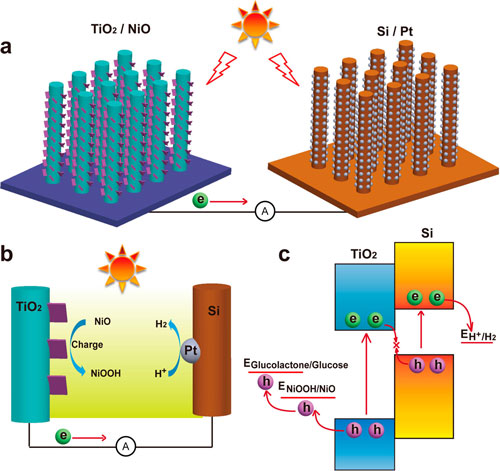
| Schematic of the TiO2/NiO photoanode and the Si/Pt photocathode for the solar-powered PEC-pseudocapacitive system. (a) Structures of the materials and devices. (b) Solar-powered PEC-pseudocapacitive mechanism. (c) Energy diagram of the system. (Reprinted with permission from American Chemical Society) | |
| With the materials for energy conversion, energy storage and energy utilization integrated together in the photoelectrodes, this device can directly utilize the solar energy input – i.e. just exposing the sensor to sunlight – to produce a chemical response to the glucose level in an aqueous solution. | |
| "Recently, there have been two very important developments in the fields of solar energy conversion and electrical energy storage," Zheng explains the background for this work. "First, a solution-grown TiO2/Ni(OH)2 nanocomposite has been reported to exhibit integrated solar hydrogen production and pseudocapacitive energy storage, in which the oxidative energy is stored by chemical conversion of Ni2+ into Ni3+ ("Integrated photoelectrochemical energy storage: solar hydrogen generation and supercapacitor")." | |
| In this work, though, due to the relatively low conduction band minimum of TiO2, an external electrical field is necessary to drive the electron flow for the water reduction, which does not yet meet the goal of direct energy conversion and storage. | |
| "Second" continues Zheng, "the recent substantial development of artificial photosynthesis approaches suggests that using two semiconductor light absorbers, with band diagrams configured as the 'Z-scheme', provides an effective approach to cover a larger part of solar spectrum for enhanced photoabsorption, as well as allows for efficient reduction and oxidation at each photoelectrode (see for instance: Light-Induced Charge Transport within a Single Asymmetric Nanowire and "A Fully Integrated Nanosystem of Semiconductor Nanowires for Direct Solar Water Splitting"). | |
| Inspired by these two important developments, the team's work was able to demonstrate the use of combined semiconductor materials and metal catalysts for efficient solar photoelectrochemical conversion and electrochemical energy storage/utilization. | |
| "Distinct from the previous report of electric field-biased device, our material system fully relies on the solar energy for charge carrier separation and transport, and provides selective targeting of glucose analytes in aqueous solution with high sensitivity," Zheng points out. "our work is the first time that a photoelectrochemical conversion is coupled with pseudocapacitive energy storage, without the assistance of additional electrical bias voltage. In addition, it is the first time that an electrochemical sensor is fully driven by solar energy, without any signal amplification methods." | |
| This work has two specific potential applications. Firstly, the enhanced solar energy conversion integrated with direct energy storage suggests new device design/structures for efficient solar power utilization. Secondly, the direct use of solar energy for glucose sensing, without the need of any auxiliary instruments, offers a convenient point-of-care detection method that can be used widely at home or office, thus serving as a fast, sensitive diagnosis of diabetics and other diseases. | |
| Zheng notes that it has been widely accepted that any single material cannot fulfill the goal of efficient solar energy utilization. "The future direction of this research field still requires the further development of new structure design and synthetic methods for realizing hybrid material and catalyst composites that can enhance their solar conversion and electrical energy storage efficiency," he says. "One particular challenge is to find the material combination that offers the most optimal photoabsorption, band gap alignment to water and chemical targets of interest, charge transport, and surface chemical reaction kinetics." | |
| "The other particular challenge is to increase the material load and stability of the photoelectrode for large capacity, long cycling life devices, as well as using all earth-abundant materials for reducing the fabrication cost." | |
| Zheng's co-authors are Yongcheng Wang, Jing Tang, Zheng Peng, Yuhang Wang, Dingsi Jia, Biao Kong, Ahmed A. Elzatahry, and Dongyuan Zhao. This work was supported by the National Key Basic Research Program of China, the Natural Science Foundation of China, and the Deanship of Scientific Research of King Saud University. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
