| Posted: Mar 30, 2015 | |
Permselective graphene oxide separator for very stable lithium-sulfur batteries |
|
| (Nanowerk Spotlight) The chemistry of widely used lithium-ion batteries limits how much energy they can store. Having been researched and developed intensely over the past decades, this mature technology has reached its theoretical limit. One promising alternative is the lithium-sulfur battery – employing sulfur as cathode and metallic lithium as anode materials – which theoretically can render 3-6 times higher energy density (2600 Wh kg-1) than lithium-ion batteries. | |
| The use of sulfur as a cathode material has advantages with regard to environmental benignancy, natural abundance, low cost, and a wide operating temperature range. However, the multi-electron-transfer redox chemistry of lithium-sulfur systems induce the formation of polysulfide intermediates, which may cause severe side reactions in lithium sulfur cells. | |
| An electrochemical energy storage system (i.e. a battery) commonly includes electrodes, electrolyte, and separator. The electrolyte systems contribute to the ionic connection between cathode and anode, while the separators serve as electronic insulators to prevent the short circuit between anode and cathode. | |
| The commonly used separators in battery systems are porous polymer membranes, which separate the two electrodes while having little impact on the transportation of ions through the membrane. Polysulfides generated in a lithium-sulfur system can also diffuse freely through the membranes and react with a metal lithium anode, which results in the degradation of the battery's performance. | |
| "If a novel, ion-selective but highly permeable separator can be developed, the shuttling of polysulfides and self-discharge would be effectively prevented, and both the energy density and power density of lithium-sulfur batteries could be significantly improved," Dr. Qiang Zhang, an associate professor at the Department of Chemical Engineering at Tsinghua University, tells Nanowerk. | |
| By introducing ion-selective membranes of ultrathin graphene oxide (GO) as separator systems, Zhang and his collaborators created a high-stable and anti-self-discharge lithium-sulfur cell. The team reported their findings in ACS Nano ("Permselective Graphene Oxide Membrane for Highly Stable and Anti-Self-Discharge Lithium-Sulfur Batteries"). | |
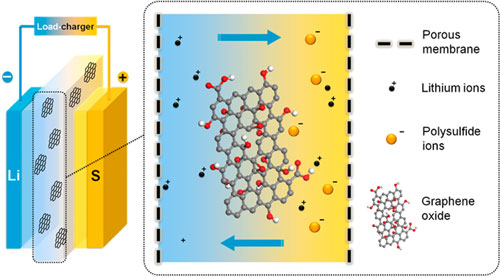 |
|
| Schematic of GO membrane incorporates in a lithium-sulfur battery. GO membrane was sandwiched between cathode and anode electrodes, which efficiently prohibited the shuttle of polysulfides through the membrane. (Image: Dr Qiang Zhang, Tsinghua University) | |
| "Highly soluble polysulfide intermediates, in the form of chain-like polysulfide anions, are present in the electrolyte of a conventional lithium-sulfur cell," Zhang explains the reason why lithium-sulfur cells have poor life spans. "The polysulfides are generated at the cathode side, diffuse through the membrane, react with lithium anode, and shuttle back. During the whole process, polysulfides dissolve and irreversibly react with metal lithium and organic components, inducing the destruction of the cathode structure, depletion of the lithium anode, and loss of active sulfur materials." | |
| "As one of the most attractive macroscopic forms of graphene-based materials, graphene oxide membranes are inherently of good mechanical strength and may stand on its own," notes Dr. Jia-Qi Huang, first author of the paper. "Enlightened by the recent report on GO membrane allows unimpeded permeation of water and rapid diffusion of smaller ions through the membranes by Geim and co-workers ("Unimpeded Permeation of Water through Helium-Leak-Tight Graphene-Based Membranes"), as well as an ion selective membrane for alkali and alkaline earth cations reported by Zhu and co-workers ("Selective Trans-membrane Transport of Alkali and Alkaline Earth Cations through Graphene Oxide Membranes Based on Cation-π Interactions"), we have had the idea to use GO membrane as permselective separator for lithium-sulfur batteries." | |
| He points out that, on one hand, GO separators are able to block polysulfide by electrostatic repulsion and steric exclusion, which are more efficient in long-term inhabitation of the shuttle effect. On the other hand, the typical two-dimensional structure of GO flakes can reduce the loading amount to form an effective polysulfide shield layer. | |
| "We applied the cationic-permselective GO membrane in a lithium-sulfur cell with the expectation that the anions of polysulfides are confined to the cathode side," recounts Huang. "This would significantly suppress the shuttle of polysulfides between cathode and anode sides. Meanwhile, the lithium cations can easily transport through the cationic-permselective GO membrane, which guarantees a high capacity and good rate performance of the lithium-sulfur cell." | |
| The team's experiments showed that, indeed, with the incorporation of a permselective GO membrane, the lithium-sulfur batteries afforded an improved Coulombic efficiency from 67-75% to over 95-98% at 0.1 C. The cyclic capacity decay rate was also reduced from 0.49 to 0.23% per cycle. | |
| "This ultrathin GO separator also offered remarkable improvement in the cyclic stability and self-discharging inhibition of lithium-sulfur batteries," says Ting-Zhou Zhuang, a co-author of the paper. "The open circuit voltage of a normal cell with a conventional membrane decays drastically from 2.5 to 2.18 V in 5 hours. When we employed the GO membrane as a separator, the open circuit voltage remained at 2.5 V for more than 30 hours due to the prevention of high-order polysulfides diffusion. This indicated the self-discharging was significantly inhibited with the incorporation of GO membrane." | |
| This work shows that a GO membrane with highly tunable functionalization properties, high mechanical strength, low electric conductivity, and facile fabrication procedure, is an effective permselective separator system in lithium-sulfur batteries. | |
| "The oxygen electronegative atoms modified GO into a polar plane and the carboxyl groups acted as ion hopping sites of positively charged species (Li+) while rejected the transportation of negatively charged species (Sn2-) due to the electrostatic interactions," explains Huang. "Such electrostatic repulsion and physical inhibition largely decreased the transference of polysulfides across the GO membrane in the lithium-sulfur system." | |
| This work offers a general strategy to integrate GO membranes into rechargeable battery systems, which is crucial for illustrating the potential of functional membranes for advanced energy storage and understanding the dynamic changes on the electrode. | |
| "Such an approach may also provide a novel cell configuration applicable to lithium-air batteries and fuel cells, which require delicate control of ion transport for high performance devices," concludes Zhang. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
