| Posted: Jun 17, 2015 | |
Synthesizing a boron-rich cousin of graphene |
|
| (Nanowerk Spotlight) The discovery of graphene, carbon's two-dimensional (2-D) allotrope, has revolutionized the technologies of energy storage devices, diagnostic sensors, optoelectronics, and ultra-strong composites. The large interfacial area combined with a nanoscale thickness (∼20,000 times thinner than human hair) impart several fascinating attributes to graphene. The ability of carbon to exist in the form of free standing graphene has motivated researchers to investigate if elements other than carbon can also form similar low-dimensional planar allotropes that would potentially exhibit properties at the extremes of all known materials. | |
| While exploring the possibility to realize graphene-like nanostructures of boron, carbon's neighbor in the periodic table, a team of chemical engineers at the Indian Institute of Technology Gandhinagar (IITGN) has discovered an entirely new family of 2-D compounds. | |
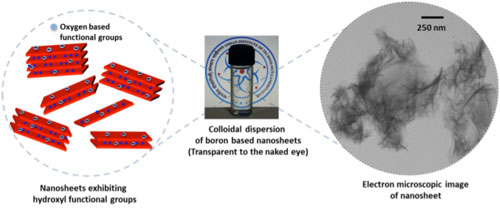 |
|
| The nanosheets exfoliated from metal borides resemble graphene, are rich in boron, and carry oxy-functional groups. The aqueous dispersions looks completely transparent as these nanosheets don’t absorb in the visible regime. (Image: Saroj Kumar Das, IITGN) (click on image to enlarge) | |
| Their research team, led by Dr. Kabeer Jasuja, Assistant Professor of Chemical Engineering at IITGN, has demonstrated exfoliation of a well-known superconductor Magnesium diboride (MgB2), a layered material that consists Mg atoms sandwiched in between born honeycomb planes. | |
| The team's findings have been recently published in an article titled "Aqueous dispersions of few-layer-thick chemically modified magnesium diboride nanosheets by ultrasonication assisted exfoliation" in Scientific Reports. | |
| The element Boron presents an extremely curious case in its ability to be structured as graphene. While it can't independently form a graphene like structure, it forms a graphenic arrangement in association with electron donor elements. This is exemplified in Magnesium diboride which contains boron honeycomb planes alternated by Mg atoms. | |
| "The structural resemblance of MgB2 to intercalated graphite and the presence of boron honeycomb planes led our research group to explore if it can also be peeled off in a way similar to graphite to yield nanosheets which can enable access to the planar form of Boron," Jasuja tells Nanowerk. | |
| His team of researchers, including doctoral student Saroj Kumar Das, master's student Amita Bedar, and undergraduate student Aadithya Kannan, placed magnesium diboride in water and applied high frequency sound waves to peel off the layers. This resulted in partial displacement of the weakly bonded Mg atoms which loosened the parent layered structure to yield boron-rich nanosheets with micrometer scale lateral dimensions and a thickness of ∼5 nm. | |
| While being peeled off, the nanosheets also acquired the hydroxyl groups from water in exchange of Mg atoms. | |
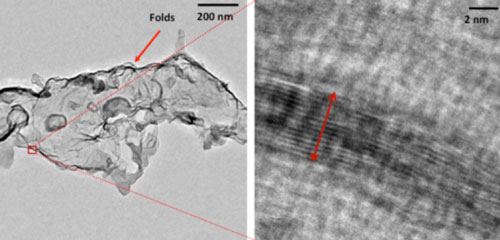 |
|
| High Resolution Transmission Electron Microscopic Image of a selected edge displaying the few layer thick attribute of the exfoliated MgB2 nanosheets. (Image: Asha Liza James, IITGN) | |
| "This decoration with hydroxyl groups enables the nanosheets to remain dispersed stably in the aqueous medium. Moreover, these groups also impart photoluminescence to these nanosheets by creating several new electron states, making the nanosheets distinctly different from the parent material," explains Saroj Kumar Das, doctoral candidate and first author of the article. | |
| While characterizing the fundamental properties of these nanosheets, the research team made an interesting observation about the transparency of the exfoliated MgB2 nanosheets: they found these nanosheets to be an order of magnitude more transparent compared to their cousin graphene, which makes the aqueous dispersion of these nanosheets appear completely transparent to the naked eye. | |
| "These chemically modified MgB2 nanosheets present excellent constructs for leveraging the rich chemistry of boron," concludes Jasuja. "The ability to exfoliate MgB2 has provided an entirely new perspective to the science of metal borides. It will be extremely promising to explore the exfoliation of other members from the large family of metal borides which are known to exhibit a range of extraordinary properties in their native form including high stabilities, ultra-high strengths and excellent thermo-electric properties. This study forms a first foundational step towards exfoliating other metal borides." | |
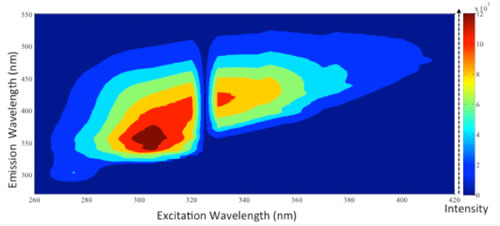 |
|
| A map of photoluminescence intensity for the exfoliated MgB2 nanosheets that exhibit excitation wavelength dependent photoluminescence. (Image: Saroj Kumar Das, IITGN) (click on image to enlarge) | |
| Prof. Kabeer Jasuja is recently joined by Asha Liza James, a doctoral student in chemical engineering, who is designing an extremely innovative chemical recipe to peel off the layers in metal borides; and master's student Gunda Harini, who is utilizing the insights from the exfoliation of MgB2 to synthesize Boron-rich quantum dots. | |
| Their research is being supported by the seed grant funding from IITGN, Start Up Research Grant for Young Scientists and Inspire Faculty Award Research Grant from Department of Science & Technology, India. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
