| Posted: Sep 12, 2017 | |
Alkaline earth metal vanadates: new anode materials for high performance sodium-ion batteries |
|
| (Nanowerk Spotlight) Sodium-ion battery, as an emerging battery technology beyond lithium-ion battery, has attracted great research interests recent years. Sodium-ion batteries have a similar configuration and electrochemical reaction processes with lithium-ion batteries. But the Na resources are much more abundant and cost-effective than Li resources, which makes sodium-ion batteries highly promising as next-generation energy storage devices, especially for large-scale energy storage. However, the practical application of Na-ion batteries is still not currently realized. | |
| Finding suitable electrode materials, especially anode materials, is a very important task for the development of sodium-ion batteries. "The larger ionic size of Na+ than Li+ results in a significantly different insertion reaction process. Hence the commercialized graphite anode for Li-ion battery cannot be used in Na-ion battery." says Liqiang Mai, a professor at the State Key Laboratory of Advanced Technology for Materials Synthesis and Processing, Wuhan University of Technology (WUT). | |
| In the past five years, large numbers of research works about Na-ion battery anodes have been reported. The previously reported anodes mainly include the following categories: hard carbon, Ti-based anodes, conversion reaction based anodes and alloying reaction based anodes. However, these anodes still face some challenges for commercialization. | |
| For example, hard carbon is limited by a poor rate capability and safety issues; Ti-based anodes generally display low capacity due to limited intercalation sites; other anode materials based on conversion or alloying reactions can deliver high initial capacity, but the inevitable large volume change during the discharge/charge process is a serious issue. | |
| In this case, finding new anode materials with intrinsically good electrochemical properties are still very important for the development of SIBs. | |
 |
|
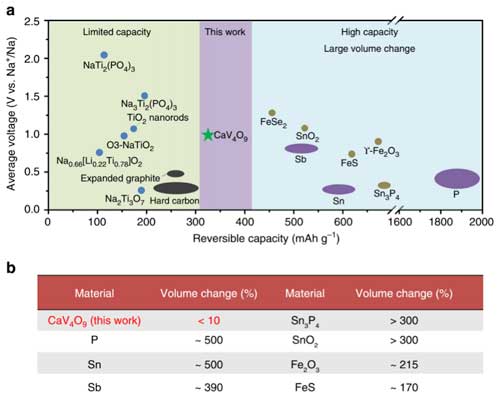
| Comparison of the CaV4O9 nanowires and previously reported SIB anodes. (a) Average voltage vs. reversible capacity of the anodes for SIBs. (b) Volume change of the reported alloying or conversion reaction anodes for SIBs. (Reprinted with permission by Nature Publishing Group) (click on image to enlarge) | |
| In new work, Mai and coworkers identified a new promising material system, the alkaline earth metal vanadates, as sodium-ion battery anodes for the first time. Their new finding has been published in Nature Communications ("Alkaline earth metal vanadates as sodium-ion battery anodes") in September 6, 2017. | |
| "Chinese traditional philosophy tells us, everything has two opposite sides, like day and night, cation and anion, electron and nucleus, and a binary cooperation between the two opposite sides will make a wonderful thing" says Xiaoming Xu, a Ph.D candidate in Mai group. "I think this thought can be also applied in electrode material design." This was the initial idea that came to Xu’s mind. | |
| "Take CaV4O9 phase as an example, which was focused by us in this work, it is an interesting material; Ca is inactive while V is active in electrochemistry; two opposite sides were combined into one material" notes Mai. "To facilitate our fundamental research about the intrinsic properties and reaction mechanism, we fabricated CaV4O9 nanowires in this work, since the unique advantage of nanowires for in situ characterization." | |
| According to the team, the first exciting property of this material is the high electric conductivity (>100 S cm-1), which is measured by assembling singe nanowire devices. Such a high electric conductivity, which is not common in electrode materials, is a basic guarantee for the good electrochemical performance. | |
| "When CaV4O9 nanowires was used as a sodium-ion battery anode, a reversible capacity over 300 mAh g-1, a rate capability up to 5000 mA g-1 (25°C) and an excellent cycling stability that up to 1600 cycles can be achieved. The comprehensive performance is much better than VO2 nanowires," Xu tells Nanowerk. "That’s very interesting; the Ca element in the sample seems to play a very important role, even though it is inactive during the electrochemical process." | |
| To reveal the internal mechanism, Mai and coworkers took advantage of in situ and ex situ TEM techniques and ab inito calculations, to investigate the detailed sodium storage mechanism both experimentally and theoretically. | |
| "We found CaV4O9 possess a very special and interesting sodium storage mechanism, different from the conventional intercalation or conversion reaction," says Mai. | |
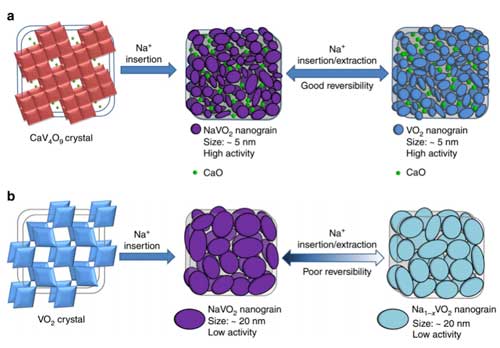
| According to the researches, the Na+ insertion into CaV4O9 will result in the decomposition of the initial structure, and NaVo2 and CaO nanograin with size of ∼5 nm will be generated. Importantly, this process exhibit very small volume change less than 10%, revealed by in situ TEM characterization and theoretical analysis. | |
| In the subsequent cycles, NaVo2 will convert to VO2 reversibly, while CaO keeps inert. However, the inactive CaO nanograins will effectively prevent the NaVo2 nanograins from agglomerating and preserve the small size of the active components, and then keep the electrodes with high reversibility. | |
| "Size plays a key role," notes Mai. "CaO nanograins help to preserve the small size of NaVo2 during the charge/dishcage cycling, and the small size of NaVo2 benefits to the Na dissociation, revealed by the ab inito calculation, which benefits to the reversible conversion from NaVO2 to VO2. In comparison, the NaVo2 nanograins derived from VO2 display a larger size about 20 nm. The larger size of NaVo2 results in the poor reversibility in the subsequent cycles." | |
 |
|
| Illustration of the reaction mechanism during initial sodiation and subsequent cycles. (a) Sodium-storage mechanism of CaV4O9. (b) Sodium-storage mechanism of VO2. (Reprinted with permission by Nature Publishing Group) (click on image to enlarge) | |
| "The high conductivity, the small volume change and the self-preserving effect from CaO results in the exciting sodium storage performance of CaV4O9," concludes Mai. In future experiments, the researchers hope to further investigate other alkaline earth metal vanadates. They believe this field will be a new direction for the exploration of electrode materials for Li/Na-ion batteries or even rechargeable Zn/Mg/Ca-ion batteries. | |
|
Provided by State Key Laboratory of Advanced Technology for Materials Synthesis and Processing, Wuhan University of Technology, as a Nanowerk exclusive.
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
