| Posted: May 01, 2008 | |
Speeding up catalytic nanomotors with carbon nanotubes |
|
| (Nanowerk Spotlight) Sophisticated molecular-size motors have evolved in nature, where they are used in virtually every important biological process. In contrast, the development of synthetic nanomotors that mimic the function of these amazing natural systems and could be used in man-made nanodevices is in its infancy. Building nanoscale motors is not just an exercise in scaling down the design of a macroworld engine to nanoscale dimensions. Many factors such as friction, heat dissipation and many other mechanical behaviors are just very different at this scale – everything is constantly moving (under kinetic energy supplied by the heat of the surroundings) and being buffeted by other atoms and molecules (Brownian motion). | |
| In nature, biological motors use catalytic reactions to create forces based on chemical changes. These motors do not require external energy sources such as electric or magnetic fields. Instead, the input energy is supplied locally and chemically. Despite impressive progress over the past years, man-made nanomachines lack the efficiency and speed of their biological counterparts. New research has demonstrated that the incorporation of carbon nanotubes (CNT) into the platinum component of asymmetric metal nanowire motors leads to dramatically accelerated movement in hydrogen peroxide solutions, with average speeds of 50-60 micrometers per second. | |
| "Our study demonstrates dramatically faster and more powerful synthetic nanomotors" Dr. Joseph Wang tells Nanowerk. "Unlike existing bimetal nanowires which are slow and weak, we illustrates that the incorporation of carbon nanotubes into such motors results in dramatic acceleration and higher efficiency. These new capabilities offer great promise to the use of synthetic nanomachines, approaching those of biological nanomotors." | |
 |
|
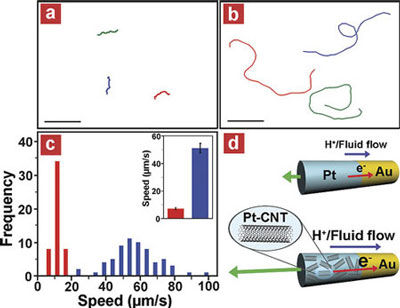
| CNT-induced high speed catalytic nanomotors: (a, b) Tracking lines illustrating a typical motion and moving distances of Au/Pt (a) and Au/Pt-CNT (0.50 mg/ml) (b) nanomotors during a period of 4 s in the presence of 15 wt % hydrogen peroxide fuel. Scale bar is 45 µm. (c) Histograms of average speeds of Au/Pt (red) and Au/Pt-CNT (blue) nanomotors measured from the movement of the nanomotors in a 15 wt % hydrogen-peroxide fuel over a 10 s period. Bar graphs with Y error bars (inset) represent the mean of average speeds (µm/s) and the error limit at 90% confidence interval of the corresponding nanomotors, respectively. (d) A schematic representation of the self-electrophoresis mechanism of Au/Pt (top) and Au/Pt-CNT (bottom) bipolar nanomotors. Hydrogen peroxide fuel is preferentially consumed/oxidized on the Pt (top, blue) or Pt-CNT (bottom, patterned blue) ends while oxygen is catalytically reduced on the Au (yellow) segment. The flux of electrons inside the nanomotors proceeds from one end to the other generating a local electric field, as well as the migration of protons and surrounding fluid outside the nanomotors resulting in the movement of the nanomotor in the opposite direction. The higher electrocatalytic activity of Pt-CNT compared with Pt provides a faster reaction rate, and hence a higher proton and fluid flow corresponding to an increased flux of electrons inside the nanomotors as indicated by the vectors. (Reprinted with permission from American Chemical Society) | |
| Wang is a professor with a joint appointment in the departments of Chemical & Material Engineering in the Ira A. Fulton School of Engineering and Chemistry and Biochemistry in the College of Liberal Arts and Science at Arizona State University (ASU). He is the Director of ASU's Center for Bioelectronics and Biosensors - The Biodesign Institute. | |
| He and his team have demonstrated a dramatic acceleration of self-powered bimetal nanomotors based on the incorporation of CNT into the platinum segment of gold/platinum nanowires. They reported their findings in the April 24, 2008 online edition of ACS Nano "Carbon-Nanotube-Induced Acceleration of Catalytic Nanomotors". | |
| "Such CNT-induced acceleration of catalytic nanomotors reflects the enhanced oxidation of the hydrogen peroxide fuel" Wang explains. "We also illustrated that the speed of nanomotors can be further increased upon adding hydrazine to the peroxide fuel and that this efficient movement can be manipulated magnetically." | |
| The researchers were able to observe further acceleration to 94 µm/s – with some motors moving above 200 µm/s – upon adding hydrazine to the peroxide fuel. | |
| Wang notes that current studies in his laboratory aim at understanding the underlying mechanisms and forces involved in these accelerated nanomotors and exploring new energy-rich chemical reactions based on different choices of fuels and a variety of motor compositions. | |
| "For example, recent experiments indicate a similar acceleration upon doping the CNT into a palladium (anodic) segment instead of a platinum one" he says. "We expect that these studies will lead to even more energy-efficient nanomotors and will open up new opportunities for nanoscale vehicle systems." | |
| Such high-performance nanomotors should allow transport and release of 'heavy' loads, locomotion in physiological conditions, and the design of more sophisticated nanosystems performing multiple complex tasks. | |
| Preliminary data compiled by the ASU team indicate that the new CNT-doped nanomotors are still self-motile when loaded with particles more than 10 times their size. | |
| Wang's team has also accomplished controlled motion in microfluidic channels (these results will be reported separately). "Currently, we are designing nanomotor-based sensing systems for monitoring the levels of fuels, including the biosensing of glucose based on the direct speed-concentration correlation" Wang describes the team's next steps. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
