| Posted: Jul 02, 2008 | |
Engineering protein-based smart materials for nanomechanical applications |
|
| (Nanowerk Spotlight) Elastomeric (i.e. elastic) proteins are able to withstand significant deformations without rupture before returning to their original state when the stress is removed. Consequently, these proteins confer excellent mechanical properties to many biological tissues and biomaterials. Depending on the role performed by the tissue or biomaterial, elastomeric proteins can behave either as springs or shock absorbers. Recent scientific work in Canada resulted in the engineering of the first artificial chameleon elastomeric proteins that mimic and combine these two different behaviors into one protein. Under the regulation of a molecular regulator, these designer proteins exhibit one of the two distinct mechanical behaviors – spring or shock absorber – which closely mimic the two extreme behaviors observed in naturally occurring elastomeric proteins. | |
| "Smart materials that can change their behaviors in response to environmental changes have always been of interests to us," Dr. Hongbin Li tells Nanowerk. "Our discovery that the mechanical stability of the small protein GB1 can be regulated by the binding of an antibody fragment provided us the opportunity to use protein engineering techniques to turn GB1 into a chameleon protein, which can display two different elastic behaviors in response to environmental stimuli." | |
| Li, an Assistant Professor and Canada Research Chair in the Department of Chemistry at the University of British Columbia (UBC) in Vancouver, Canada, reports these results – which provide a new concept to tune the mechanical performance of proteins at single molecule level – in the June 29, 2008 online edition of Nature Nanotechnology ("Engineered elastomeric proteins with dual elasticity can be controlled by a molecular regulator"). | |
| Elastic proteins are important structural and functional components in living cells. They serve as molecular springs in tissues to establish elastic connections and provide mechanical strength, elasticity, and extensibility. They are not only important for their biological functions in various biological processes, but also important building blocks for bottom-up construction of smart materials and mechanical devices on the nanoscale. | |
| "Our recent work represents a step forward for the bottom-up construction of smart mechanical materials," says Li. "Tuning the mechanical properties of proteins in a reversible fashion has been a challenging task for scientists." | |
 |
|
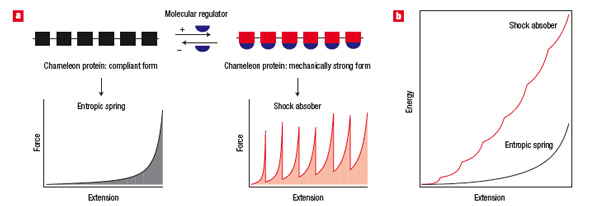
| Schematic illustration of the general concept of elastomeric chameleon proteins. a, Elastomeric chameleon proteins show dual elasticity. They can be reversibly switched between a compliant entropic spring and a mechanically stable shock absorber by a molecular regulator. The shaded areas under the force/extension curves show the energy required to stretch the protein to the given extension. b, Comparison of the energy cost for stretching an entropic spring and a shock absorber protein to the same extension. (Reprinted with permission from Nature Publishing Group) | |
| He notes that his group had previously demonstrated that protein-protein interactions can be used to tune the mechanical stability of proteins ("Protein–Protein Interaction Regulates Proteins’ Mechanical Stability"). | |
| In the above work, using single-molecule atomic force microscopy, the team showed that the binding of fragments of IgG antibody to a small protein, GB1, can significantly enhance the mechanical stability of GB1. The regulation of the mechanical stability of GB1 by IgG fragments is not through direct modification of the interactions in the mechanically key region of GB1; instead, it is accomplished via the long-range coupling between the IgG binding site and the mechanically key region of GB1. Although the Fc (hFc) and Fab fragments bind GB1 at different regions of GB1, their binding to GB1 can increase the mechanical stability of GB1 significantly. | |
| "However" explains Li, "both protein-protein interactions and mechanical stability of proteins are sensitive to structural alternation of proteins. It was unknown whether these two properties can be tuned independently in the same protein. In our paper, we successfully demonstrated that re-engineering the regions of a protein that is responsible for the mechanical stability has little effect on its interaction with another protein. Therefore, the resulted proteins can display dual mechanical properties under the regulation of protein-protein interactions." | |
| In their experiments, the scientists used the technique of proline mutagenesis to construct a mutant protein (GV54P) where the mechano-active site is disrupted but the binding affinity to hFc is retained. | |
| "Our results demonstrate that GV54P exhibits two distinct mechanical compliances depending on the presence of hFc," says Li. "In the absence of hFc, GV54P is mechanically compliant and functions as an entropic spring; however, upon binding of hFc, GV54P exhibits significant mechanical stability and unfolds sequentially upon stretching." | |
| While the UBC team's study is fundamental in nature, the concept it illustrates may help engineer protein-based smart materials for nanomechanical and biomedical applications. One example that Li mentions is that it would be possible to engineer smart hydrogels made of such elastomeric proteins that can change their mechanical and physical properties upon binding of regulator molecules. | |
| Single molecule force spectroscopy and protein engineering have made it possible to engineer proteins of well-defined mechanical properties. Designing proteins that are sensitive to environmental stimuli, like the chameleon proteins engineered by Li and his group, will be of tremendous importance for the future applications of elastomeric proteins. | |
| "The technical challenge we are facing now is the high molecular weight of the molecular regulator (IgG antibody fragment)," says Li. "The high molecular weight of IgG antibody fragments makes it difficult to using high concentrations of it to effectively regulate the mechanical properties of proteins. Currently we are experimenting different techniques to design chameleon elastomeric proteins that can be regulated by small molecules, such as metal ions." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
