| Posted: Jan 23, 2009 | |
Synthesizing colloidal 'molecules' of various shapes |
|
| (Nanowerk Spotlight) Colloidal materials have been playing a major role in many research fields such as chemistry, materials science, condensed matter physics, applied optics, fluid dynamics, and biology. Most of these studies require the use of monodisperse colloidal particles with uniformity in shape, size, composition, structure and surface properties. | |
| "Both our current understanding of various physical phenomena and our capability to fabricate new functional materials have been considerably enriched by the development of synthetic strategies that are capable of generating copious quantities of colloidal entities of good size uniformity," Serge Ravaine explains to Nanowerk. "Nevertheless, most of the available monodisperse colloidal materials are spherical because this represents a thermodynamically favorable state in terms of surface energy. This strongly limits the number of new structures which can be engineered by using these colloids as building blocks. For instance, the crystallization of spherical colloids into three-dimensional periodic lattices has recently allowed the emergence of a very active field of research – photonic colloidal crystals, known as artificial opals. Nevertheless, the light diffraction properties of these crystals are rather limited because of their face-centered cubic lattice, which results from the packing of spheres." | |
| Ravaine, a professor of chemistry at the Centre de Recherche Paul Pascal, a laboratory of the National Center for Scientific Research (CNRS) in France, together with collaborators from various departments at the Université Bordeaux 1, has demonstrated a novel synthetic route that can produce a full set of new non-spherical, binary colloids with regular morphology in a high yield. | |
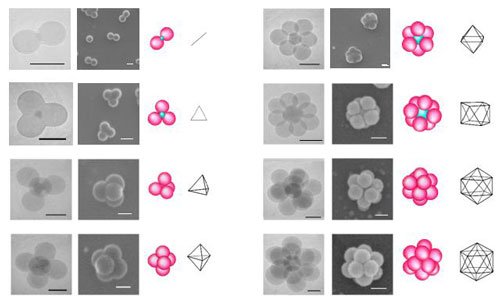 |
|
| Transmission and scanning electron micrographs of the binary clusters obtained after 2 hours with silica seeds of different diameters (left columns, scale bar: 200 nm). Schematic drawings of the sphere configurations (center columns). Polyhedra formed by drawing lines from the center of each polystyrene nodule to its neighbors (right columns). (Image: Dr. Ravaine, Centre de Recherche Paul Pascal) | |
| The French team's results, reported in Angewandte Chemie (A Chemical Synthetic Route towards "Colloidal Molecules"), offers the exciting prospect that this synthetic route can be extended to mimic all the molecules that have a single central atom: not only incomplete morphologies such as bent geometries (H2O or SnCl2), trigonal pyramids (NH3), but also multinuclear molecules whose central atom is combined with more than two atom types. | |
| "Indeed" says Ravaine, "the experimental parameters, which can be easily varied, are the nodule-to-seed ratio, the seed nature, the polymerization time (for adjusting the nodule size to the seed size) and the successive addition of monomers of different natures. The scalability of this technique would allow the preparation of colloids in amounts sufficient for studying their interactions in water and their packing to create original materials such as photonic crystals with a full bandgap." | |
| The main components of the fabrication process employed by Ravaine's team were uniformly sized silica beads. These seeds were the basis for an emulsion polymerization of a monomer that resulted in colloidal particles with a well controlled morphology. A key step in the process was the surface modification of the silica seeds with methacryloxymethyltriethoxysilane (MMS). | |
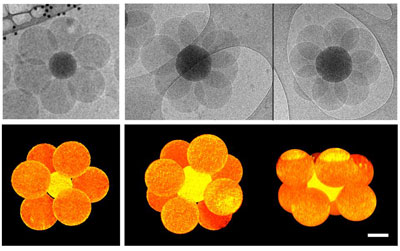 |
|
| Top row: hexapod-like and octopod-like silica/polystyrene clusters as observed by cryo-TEM after 2 hours of polymerization from silica seeds. Bottom row: 3D tomographic reconstructions of the same silica/polystyrene clusters revealing they are octahedra (left) and square antiprisms, respectively. Scale bar: 100 nm. (Image: Dr. Ravaine, Centre de Recherche Paul Pascal) | |
| The scientists also showed that the morphologies of the particles result from the minimization of an energy term that is the sum of two forces – an attraction towards the center and two-body particle repulsions that balance the attractive force. | |
| "This minimization principle provides not only some insights into the mechanism of the constrained growth of polymer nodules onto the surface of seeds, but it also can be viewed as an analogue at the mesoscopic scale of the valence shell electron pair repulsion (VSEPR) model allowing to predict the molecular geometry," says Ravaine. | |
| Using the 'colloidal molecules' as building blocks to fabricate functional materials is still a considerable challenge, but one the French team would like to overcome. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
