| Posted: Mar 22, 2007 | |
Nanotechnology could lead to improved implant devices |
|
| (Nanowerk Spotlight) The market for medical implant devices in the U.S. alone is estimated to be $23 billion per year and it is expected to grow by about 10% annually for the next few years. Implantable cardioverter defibrillators, cardiac resynchronization therapy devices, pacemakers, tissue and spinal orthopedic implants, hip replacements, phakic intraocular lenses and cosmetic implants will be among the top sellers. Current medical implants, such as orthopaedic implants and heart valves, are made of titanium and stainless steel alloys, primarily because they are biocompatible. | |
| Unfortunately, in many cases these metal alloys with a life time of 10-15 years may wear out within the lifetime of the patient. They also might not achieve the same fit and stability as the original tissue, and in a worst case, the host organism might reject the implant altogether. | |
| While available implants can alleviate excruciating pain and allow patients to live more active lives, there often are problems getting bone to attach to the metal devices. Small gaps between natural bone and the implant can increase over time, requiring the need for additional surgery to replace the implant. In the quest to make bone, joint and tooth implants almost as good as nature's own version, scientists are turning to nanotechnology. | |
| Researchers have found that the response of host organisms (including at the protein and cellular level) to nanomaterials is different than that observed to conventional materials. While this new field of nanomedical implants is in its very early stage, it holds the promise of novel and improved implant materials. | |
| There is a huge demand for tissue regeneration technologies, which covers a wide range of potential applications in such areas as cartilage, vascular, bladder and neural regeneration. Just consider the need for bone and dental implants: Each year, almost 500,000 patients receive hip implants worldwide, about the same number need bone reconstruction due to injuries or congenital defects and 16 million Americans loose teeth and may require dental implants. | |
| There seems to be growing consensus among scientists that nanostructured implant materials may have many potential advantages over existing, conventional ones. | |
| "We continuously are finding more and more positive attributes of using nanomaterials as improved orthopedic implants" Dr. Thomas J. Webster tells Nanowerk. "Our results point to the idea that no matter what material chemistry one is interested in (metals, ceramics, polymers, composites), increased bone formation can be achieved by nanostructuring these materials. This can be done by using a number of manufacturing techniques including by using nanoparticles themselves, e-beam evaporation, chemical etching, or lithography. In some cases, we see a three to five times faster regeneration of bone on materials of the same chemistry but with nano compared to micron grain sizes." | |
| Webster is an associate professor for the Division of Engineering and Orthopaedics at Brown University. Together with Dr. Ganesan Balasundaram, also at Brown, he published a review article that discusses recent studies that have been conducted to determine the efficacy of nanostructured materials as orthopedic implants ("A perspective on nanophase materials for orthopedic implant applications"). | |
| Previously, Webster had written a wider ranging review covering nanomedical implants in general ("Nanomedicine for implants: A review of studies and necessary experimental tools"). | |
| Webster outlines the requirements for successful bone implants: They should not only temporarily replace missing bone but provide a framework into which the host bone and vascular network can regenerate and heal; they should act as a scaffold to support new bone formation, blood vessels and soft tissue as they grow to connect fractured bone segments; and, ideally, the implant should also interact with the host tissue, recruiting and even promoting differentiation of osteogenic cells, rather than acting as a passive stage for the performance of any itinerant cells. | |
 |
|
 |
|
 |
|
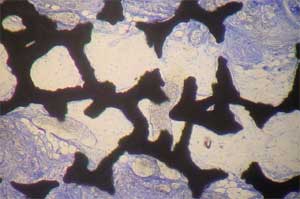
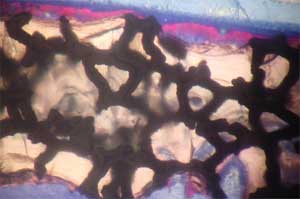
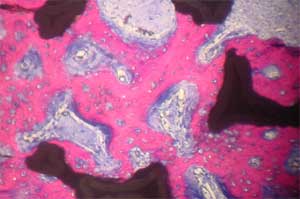
| Histology of rat calvaria (the skullcap) after tantalum (Ta) scaffolds coated with either nanophase or conventional hydroxyapatite (HA) were implanted for 2 weeks. Red shows new bone infiltration which occurred in greater amounts on nano-HA-coated Ta than on either conventional HA-coated Ta or uncoated Ta. Top: Uncoated Ta scaffold; Middle: Conventional HA coated Ta scaffold; Bottom: Nano-HA coated TA scaffold (higher magnification) (Images: Thomas J. Webster/Brown University) | |
| "There are clearly two important – but not unrelated – factors for orthopedic implant material selection" says Webster: "the correct chemistry to support or stimulate an appropriate host response and the geometric engineering of an appropriate scaffold structure." | |
| He points out that there has been a history of "trial-and-error" approaches for orthopedic material chemistries, which have not overwhelmingly improved the success of bone implants. Frequently, implant materials promote the formation of undesirable soft connective tissue by other cells such as fibroblasts. | |
| "It has become clear that to design better orthopedic implant materials, we need to concentrate on cellular processes that lead to efficient new bone growth" says Webster. "The efficacy of bone regeneration is determined mainly by surface characteristics such as the chemical composition and physical properties of the implant that controls initial protein adsorption." | |
| Rather than just experimenting by trial and error, scientists are aiming to intelligently design implant surfaces to control protein interactions important for subsequent cell adhesion that may provide answers to those problems which have plagued current orthopedic implants. | |
| Three factors have become key areas in the development of improved orthopedic devices: topography, where nanoscale surface structuring would optimize cell colonization; surface chemistry, where scientists attempt to control and optimize the chemical surface properties of an implant material; and wettability, due to the observation that cell adhesion and subsequent activity are generally better on hydrophilic surfaces. | |
| For instance, studies imply that cell responses might be more sensitive to changes in surface roughness features in the nanometer (<100 nm) compared with conventional (>100 µm) regimes and sensitivity may vary with cell type. | |
| Surface structuring and chemistry modification would require nanoscale processes while engineered nanomaterials would play a role in increasing wettability. "For example," says Webster, "we have demonstrated that aqueous contact angles were three times smaller (i.e., more wettable) when alumina grain size was decreased from 167 nm to 24 nm." | |
| Although nanostructured implant materials may have many potential advantages in the context of promoting bone cell responses, it is important to remember that studies on nanophase materials have only just begun. | |
| Webster cautions that "there are still many other issues that must be answered. Most importantly, influences of nanoparticulates on human health are not well understood, whether exposure occurs through the manufacturing or through the implantation of nanophase materials. Clearly, detailed studies in this context are required if nanoparticles are to be used in implant systems." | |
| For instance, nanoparticles could become loose through the degradation of implanted polymeric materials or they could be generated at artificial joints where friction between two surfaces is high. While the outcome of micron-sized wear debris on bone health has been well-studied for several decades, the influence of nanoparticulate wear debris (or nanoparticles in general) on bone cell health is only just beginning to be understood. | |
| Despite the challenges that lie ahead, Webster is optimistic: "Significant evidence now exists elucidating that nanophase materials represent an important growing area of research that may improve bonding between an implant and surrounding bone. It has proven to be a versatile approach that can increase bone cell functions on a wide range of orthopedic implant chemistries." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com.
