| Posted: Jan 13, 2017 | |
Preventing frost formation with nanoengineered surfaces (w/video) |
|
| (Nanowerk Spotlight) Frost and ice accumulation result in significant decreases in the performance of ships, wind turbines, and heat exchangers. The use of active chemical, thermal, and mechanical methods of ice removal is time consuming and costly. The development of passive methods to inhibit condensation, frost and ice formation is an attractive alternative. | |
| Researchers like Konrad Rykaczewski, an assistant professor at School for Engineering of Matter, Transport and Energy at Arizona State University, focus on understanding the micro- and nanoscale mechanism of frost and ice accumulation on nanoengineered anti-frost and anti-icing superhydrophobic and lubricant impregnated surfaces. | |
| Such nanoengineered surfaces possess special wetting properties that can not only efficiently repel or attract liquids like water and oils but can also prevent formation of biofilms, ice, and other detrimental crystals. | |
| Rykaczewski and his PhD student Xiaoda Sun have found a new way to inhibit condensate and frost nucleation that is compatible with icephobic coatings as well as current de-icing techniques. | |
| Reporting their findings in ACS Nano ("Suppression of Frost Nucleation Achieved Using the Nanoengineered Integral Humidity Sink Effect"), the two scientists systematically explore how frost growth can be inhibited by controlling water vapor concentration using porous bilayer coatings infused with a hygroscopic liquid, and they revealed intriguing size effects in this system. | |
 |
|
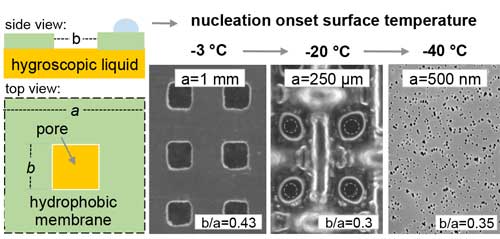
| Schematic of the nanoporous bilayer coating and results of experiments with the mesh size getting smaller. (Reprinted with permission by American Chemical Society) (click on image to enlarge) | |
| "The first step in condensation and frost growth is formation of nanoscale nuclei on the surface," Rykaczewski explains to Nanowerk. "From thermodynamics we know that there are two ways to prevent or slow nucleation: changing the surface chemistry so that molecules do not like to aggregate on it or decreasing the concentration of water molecules above the surface." | |
| He points out that the first approach is relatively easy and has been tried extensively: it basically requires a surface to be coated with something like Teflon™. Unfortunately, small defects on the surface such as little scratches can facilitate nucleation. | |
| "Instead of hoping for perfect surfaces, we decided to explore whether it would be possible to design coatings that could alter nucleation using the second approach," says Rykaczewski. "Specifically, we wanted to make a coating that would alter water vapor concentration above it." | |
| Based on their earlier work (Langmuir, "Inhibition of Condensation Frosting by Arrays of Hygroscopic Antifreeze Drops"), the researchers hypothesized that this could be achieved by engineering a process referred to as humidity sink effect. This phenomena occurs when a hygroscopic (i.e. highly water vapor absorbing) liquid is exposed to air. | |
| It has been known for a while that when a droplet of hygroscopic liquid is placed on a cold surface, condensation or frost form everywhere but within a region around the drop where the droplet 'sucks' up water vapor (see video below) One can do this experiment at home by putting a salty water drop in the freezer. | |
| Demonstration of condensation frosting inhibition on a bilayer coating infused with propylene glycol with an individual drop with diameter of 500 µm. (Video: Rykaczewski Lab) | |
| "In our previous work, we showed that by placing hygroscopic drops close enough for the nucleation free regions to overlap, condensation and frost can be prevented over the entire surface," Rykaczewski notes. "In our present paper, we used the idea of bi-layer coatings with a porous outside separating a hygroscopic liquid infused layer. Analogously to drops, when pores with the liquid are spaced close enough, nucleation does not occur on the outside." | |
| "Interestingly" he continues, "the model we developed suggests that as the size and spacing of these pores decreases to the nanoscale, the concentration above the surface becomes uniform, independent of the hole size, and equal to that set by the hygroscopic liquid. This is really intriguing: when the pore size is optimized, the system behaves as if the membrane was not there." | |
| This means that in order to nucleate, the saturation concentration and that set by the hygroscopic liquid need to be nearly equal. This happens at very low temperatures, and as a result the team did not observe any nucleation until about -40°C when the sample was in 100% water-saturated air at 25°C (i.e. the dew point is depressed by 65°C). | |
| "We proved these theoretical predictions experimentally and showed that by decreasing the pore size/spacing from millimeters to nanometers while keeping the total 'open' area the same, we can continually decrease the dew point to -40°C," says Rykaczewski. "This of course lasts only until the liquid is diluted, but from our experiments this period is long enough to get airplanes through the icing danger zone during their ascend and descent." | |
| One of the primary applications of these icephobic coating is in the aviation industry. In another previous work (Advanced Materials Interfaces, "Bioinspired Stimuli-Responsive and Antifreeze-Secreting Anti-Icing Coatings"), the team showed that bi-layer coatings infused with antifreeze can prevent multiple forms of icing while saving a lot of antifreeze solution. | |
| "What we hope for is to test our coatings on small systems, for example UAVs that are used in search and rescue missions or by the navy to look for icebergs in the arctic," notes Rykaczewski. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
