| Posted: Jul 07, 2010 | |
A simple, one-step process to fabricate three-dimensional graphene macrostructures |
|
| (Nanowerk Spotlight) Given the massive interest and rapid developments in graphene research, scientists are now convinced that the controlled preparation of graphene-based materials with hierarchical and well-defined structures will pave the way for achieving high-performance applications of graphene in various technological fields such as optoelectronics, energy storage, polymer composites and catalysis. Self-assembly techniques have become some of the most effective strategies for this purpose. | |
| Although two-dimensional (2D) self-assembly of graphene has been studied extensively from the perspectives of fundamental research and commercial applications, three-dimensional (3D) self-assembly of 2D nanoscale graphene into functional macrostructures with well-defined networks remains as a great challenge and represents an important hurdle towards practical applications. Researchers in China have now provided a solution to this problem by demonstrating the successful preparation of self-assembled graphene hydrogel via a one-step hydrothermal process. | |
| "We have successfully prepared a mechanically strong, electrically conductive, and thermally stable self-assembled graphene hydrogel with high specific capacitance by hydrothermal reduction of graphene oxide (a compound of carbon, oxygen, and hydrogen obtained by treating graphite with strong oxidizers) aqueous dispersion," Gaoquan Shi, a professor in the Department of Chemistry at Tsinghua University in Beijing, tells Nanowerk. "Our method is simple, scalable, and environmental friendly." | |
| In the June 30, 2010 online edition of ACS Nano ("Self-Assembled Graphene Hydrogel via a One-Step Hydrothermal Process"), Shi and his group report a high- performance self-assembled graphene hydrogel – the first example of self-assembling 2D graphene sheets into 3D macrostructures via a convenient one-step process. | |
| Self-assembled hydrogels are usually mechanically weak, thermally unstable and electrically insulating. The Tsinghua team discovered that graphene oxide that is dispersed uniformly in water can be reduced and self-assembled into high-performance graphene hydrogels via a facile one-step hydrothermal process. | |
| The self-assembled graphene hydrogel, which contains about 2.6% (by weight) graphene sheets and 97.4% water, has an electrical conductivity as high as 0.005 S/cm. Furthermore, it is thermally stable in the temperature scale of 25-100°C and its storage modulus (450-490 kPa) is about 1-3 orders of magnitude higher than those of conventional self-assembled hydrogels. This hydrogel used as a 3D supercapacitor electrode material exhibits high specific capacitance (175 F/g) in an aqueous electrolyte. | |
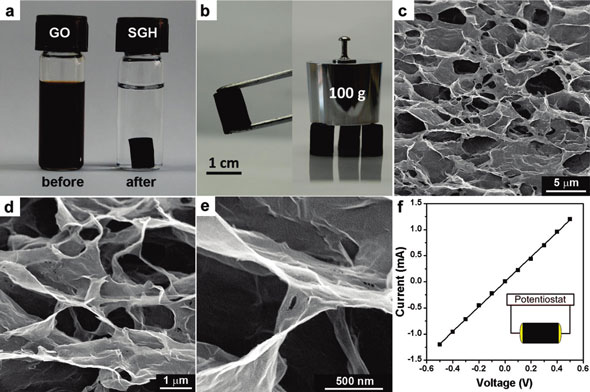 |
|
| (a) Photographs of a 2 mg/mL homogeneous graphene oxide aqueous dispersion before and after hydrothermal reduction at 180°C for 12 hours; (b) photographs of a strong self-assembled graphene hydrogel allowing easy handling and supporting weight; (c-e) SEM images with different magnifications of the self-assembled graphene hydrogel interior microstructures; (f) room temperature I-V curve of the self-assembled graphene hydrogel exhibiting Ohmic characteristic, inset shows the two-probe method for the conductivity measurements. (Reprinted with permission from American Chemical Society) | |
| Shi notes that previous research work has successfully demonstrated self-assembly of graphene building blocks into functional thin films as one important kind of graphene-based materials, such as transparent conducting membranes and strong paper-like materials, in which graphene sheets are assembled in a near-parallel manner into layered structures. | |
| "It is natural for people to think that the 2D structural nature of graphene always results in such 2D self-assembly behaviors" he says. "Therefore we set out to see if there is an effective way to self-assemble 2D nanoscale graphene into functional macrostructures with well-defined networks." | |
| Based on their experiments, the researchers propose a reasonable mechanism for the formation of self-assembled graphene hydrogel: Before reduction, the graphite oxide sheets were randomly dispersed in water and in extended states, due to their strong hydrophilicity and electrostatic repulsion effect. When the graphite oxide sheets were hydrothermally reduced, they became regionally hydrophobic, due to their restored conjugated domains and diminished oxygenated functionalities. The combination of hydrophobic and π-π interactions caused a 3D random stacking between flexible graphene sheets. | |
| "If the concentration of graphite oxide was sufficiently high, the crosslinking through partial overlapping of the flexible graphene sheets occurred timely and finally enough cross-linking sites were generated for forming a 3D network with pore sizes ranging from submicrometer to several micrometers" Shi explains the process. "Simultaneously, the residual oxygenated functional groups on the graphene sheets could entrap ample water into the graphene network under high temperature and pressure to form a graphene hydrogel. However, when the concentration of graphite oxide was low, the cross-linking would be difficult to occur timely because of the low contacting opportunity between the graphene sheets dispersed in aqueous medium. Consequently, graphite oxide sheets were hydrothermally reduced to graphene aggregates and participated as powders." | |
| The excellent mechanical, electrical and thermal properties of SGH along with inherent biocompatibility of carbon materials appears to make it attractive in a wide variety of applications, such as drug-delivery, tissue scaffolds, high performance nanocomposites and supercapacitors. | |
| Furthermore, the method developed by Shi's team is simple, environmental friendly and can be extended to the fabrication of various nanocomposites. This work is also noteworthy because it provides a deeper understanding of the self-assembly behavior of functionalized graphene as a 2D molecular building block; findings that will inspire more novel designs of hierarchical and functional materials based on graphene. | |
| "With regard to graphene as a versatile and unique building block, there is plenty of room for the discovery of new assembly behaviors of graphene itself or with other functional building blocks like macromolecules and inorganic nanoparticles for guiding the design and preparation of graphene-based functional materials" says Shi. "Given the research progress in this field, we can anticipate that self-assembled, graphene-based functional materials with high performances and even stimuli responses will appear in the near future." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
