| Posted: Jan 17, 2013 | |
Almost 250 nanomedicine products approved or in clinical study |
|
| (Nanowerk Spotlight) According to an expert group of the European Medicines Evaluation Agency Source ("Reflection paper on nanotechnology-based medicinal products for human use"), "the majority of current commercial applications of nanotechnology to medicine is geared towards drug delivery to enable new modes of action, as well as better targeting and bioavailability of existing medicinal substances. Novel applications of nanotechnology include nanostructure scaffolds for tissue replacement, nanostructures that allow transport across biological barriers, remote control of nanoprobes, integrated implantable sensory nanoelectronic systems and multifunctional chemical structures for drug delivery and targeting of disease." | |
| While most reviews on nanomedicine tend to focus on specific sectors or take a very forward-looking stance and fail to provide a complete perspective on the current landscape, a new review article in Nanomedicine: Nanotechnology, Biology and Medicine ("The big picture on nanomedicine: the state of investigational and approved nanomedicine products") provides a more comprehensive and contemporary inventory of nanomedicine products. | |
| The authors gathered information on 247 nanomedicine products that are already approved or in various stages of clinical study and describe each based on various dimensions, including FDA classification, approval status, nanoscale size, treated condition, nanostructure, and others. | |
| They point out, though, that it is difficult to locating basic information on nanomedicine products: "This is partly due to the lack of a clear definition and categorization of nanomedicine as a unique product class. However, it is also difficult to identify products that do not use nano-related terminology in their literature, and this can serve as an impetus for companies to avoid nano branding, if they perceive an elevated regulatory effort or negative public perception." | |
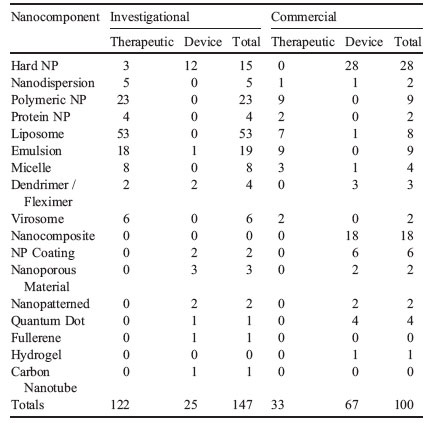
| The table below provides a breakdown of the type of nanostructures utilized in the confirmed and likely nanomedicine products. The various forms of free nanoparticles were the most prevalent categories, with significant numbers in both commercial products and investigational applications. | |
 |
|
| Type of nanostructure for confirmed and likely nanomedicine applications and products, by developmental status. (© Elsevier) | |
| The high level of development in nanoscale liposomes and emulsions should be highlighted; many developing drug-delivery platforms take advantage of liposomal and emulsion formulations. | |
| In the above table, "therapeutic" is defined to include drugs, vaccines, and biologicals that are intended to directly remedy a medical condition. All other applications and products were generally classified as "devices". | |
| Based on their documentation of the many nanomedicine products already in use in humans, the study indentifies several interesting trends forecasting the future of nanomedicine. "The most overwhelming trend observed in the data is the many cancer treatments under development. This circumstance can be tied to the significant investments the National Cancer Institute (NCI) has made in nanotechnology over the past decade, the fact that cancer is the worldwide leading cause of death, and the inherent benefits that nanoplatforms offer for therapeutic delivery. However, it might also be due in part to the sense that life-threatening cancers warrant the investigation of treatments using emerging technologies such as nanotechnology." | |
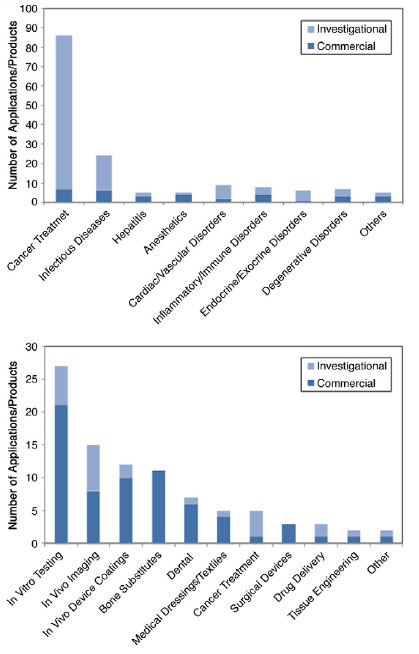 |
|
| Medical uses for confirmed and likely nanomedicine therapeutics (top) and devices (bottom) identified. (© Elsevier) | |
| Another theme playing a major role in today's nanomedicine that is likely to undergo significant development in the near future is in vivo targeting. The review identifies a large number of products utilizing the enhanced permeability and retention (EPR) effect, as well as several taking advantage of more active modes of targeting. | |
| Looking ahead, the authors project that the next phases of development in nanomedicine are likely to take advantage of combined applications in the form of both multimodal treatments (utilizing nanomedicine in combination with current treatments) and theranostic platforms (single nanomedicine applications with multiple modes of action). | |
| Based on their difficulties with clearly identifying nanomedicine applications, the authors state that this reveals two clear needs that should be addressed for any regulatory approach for nanomedicine to succeed: "1) developing an effective and clear definition outlining the field and 2) creating a standardized approach for gathering, sharing, and tracking relevant information on nanomedicine applications and products (without creating additional barriers for medical innovation). | |
| "Both the NCL and FDA are taking steps in the right direction, but broader-reaching efforts are necessary to clarify the definition of ?nanomedicine,? track key data, and facilitate coordination among agencies in this complex arena." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
