| Posted: Sep 10, 2013 | |
Nanotechnology solutions to combat superbugs |
|
| (Nanowerk Spotlight) At at a meeting of infectious disease experts in early 2012, the Director General of the World Health Organization (WHO), Dr Margaret Chan, has warned vividly that the growing threat of antibiotic-resistant bacteria may pose grave risks for society: "A post-antibiotic era means, in effect, an end to modern medicine as we know it. Things as common as strep throat or a child's scratched knee could once again kill." | |
| Chan pointed out that there is a global crisis in antibiotics caused by rapidly evolving resistance among microbes responsible for common infections that threaten to turn them into untreatable diseases. Every antibiotic ever developed was at risk of becoming useless. Figure 1 below depicts this scary trend: | |
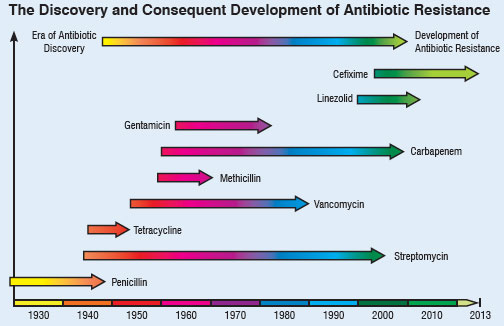 |
|
| Fig. 1: Timeline of antibiotics discovery and the development of antibiotic resistance. | |
| An antibiotic is a compound or substance that kills or slows down the growth of bacteria by different mechanisms like; 1) inhibiting the cell wall synthesis; 2) blocking DNA/RNA expression; 3) stopping the folic acid synthesis; 4) disrupting cell membrane permeability and arresting the central dogma of bacteria (DNA, RNA and protein synthesis); 5) inhibiting the protein synthesis10. ‘Antibiotic resistance’ is the resistance of micro-organism against a drug’s action, which allow them to persist in a drug-laden environment. ‘Superbugs’ embody the group of bacteria which contains several resistance genes which when expressed leads to development of antibiotic resistance by different mechanism like; 1) coding for some specific enzymes which devastate antibiotics; 2) modifying the efflux pump which causes trans-shipment of antibiotic out of the bacterial cell; 3) modifying the configuration of target site so that antibiotics cannot bind; 4) production of alternative target (enzyme) to bind the antibiotics. | |
| In the past 70 years, antibiotics have been critical in the fight against infectious diseases caused by bacteria and other microbes. Antimicrobial chemotherapy has been a leading cause for the dramatic rise of average life expectancy in the 20th century. The most important development in medicine in this century is perhaps the discovery of penicillin by Alexander Fleming in 1928, a naturally occurring antibiotic that inhibits cell wall synthesis in many pathogenic bacteria. In 1940, E. B. Chain and H. W. Florey were able to produce stable commercial formulations of this antibiotic. But just four years after drug companies began mass-producing penicillin in 1943, microbes began appearing that could resist it. | |
| The first bug that became resistant to penicillin was Staphylococcus aureus3. This bacterium is often a harmless passenger in the human body, but it can cause illness such as pneumonia or toxic shock syndrome, when it overgrows or produces a toxin. In 1967, another type of penicillin-resistant pneumonia, caused by Streptococcus pneumonia and called pneumococcus, surfaced in a remote village in Papua, New Guinea. In 1983, a hospital acquired intestinal infection caused by the bacterium Enterococcus faecium joined the list of bugs that outwit penicillin. In the past half century, from penicillin to methicillin to vancomycin to daptomycin, a large number of multiple classes of antibiotics have been discovered that inhibit cell-wall synthesis. | |
| Over the years, however, more and more microorganisms, exposed to more and more antibiotics, have adapted to these compounds. Resistance to antimicrobial drugs has now become a worldwide problem. | |
| As the use of antibiotics increases for medical, veterinary and agricultural purposes, the increasing emergence of antibiotic-resistant strains of pathogenic bacteria is an unwelcome consequence. The incidence of the multidrug resistance (MDR) of bacteria which cause infections in hospitals/intensive care units is increasing, and finding microorganisms insensitive to more than 10 different antibiotics is not unusual5. | |
| The most striking example is Tuberculosis (TB), which is one of the most deadly diseases in the world. The bacteria causing multidrug-resistant TB (MDR-TB) are resistant to the most potent anti-TB drugs like isoniazid and rifampicin. Extensively drug-resistant TB (XDR-TB) is even more deadly and resistant to any of the second-line anti-TB injectable drugs. The statistics on trends in morbidity and mortality attributed to MDR-TB, and reported by WHO is quite scary. According to them, every year about 440,000 MDR-TB cases are estimated to have appeared globally, and out of which about 150,000 patients die7. India has the highest MDR-TB burden in the world. It is estimated that about 73,000 MDR-TB cases are emerging every year. Moreover, the average cost of treating a patient with MDR-TB is estimated to be about US $9000 as compared to $19 for drug-sensitive TB8. Hospital acquired methicillin-resistant Staphylococcus aureus (MRSA) is estimated to cause ~19,000 deaths per year in the United States9. Apart from their high mortality rate, MRSA infections lead to an estimated $3 billion to $4 billion of additional health care costs per year. NDM-1 (New Delhi metallo-beta-lactamase-1), is a gene carried by bacteria, which is responsible for producing an enzyme, carbapenemase, within the bacteria making them resistant to almost all the present antibiotics. | |
Nanotechnology-based solutions against superbugs |
|
| The emergence of superbugs has made it imperative to search for novel methods, which can combat the microbial resistance. Thus, application of nanotechnology in pharmaceuticals and microbiology is gaining importance to prevent the catastrophic consequences of antibiotic resistance. Nanotechnology based approaches are advantageous to improve various preventive measures such as coatings and filtration. Similarly, diagnosis using efficient nanosensors or probes can speed up the treatment process at an early stage of disease. Nano-based drug carriers for existing antibiotics enhance their bioavailability and make them more targets specific. Also the combination of nanoparticles (NPs) along with antibiotics makes them more lethal for micro-organisms. Figure 2 highlights some of the probable mechanisms by which nanomaterials by which nanomaterials cause bacterial cell death. | |
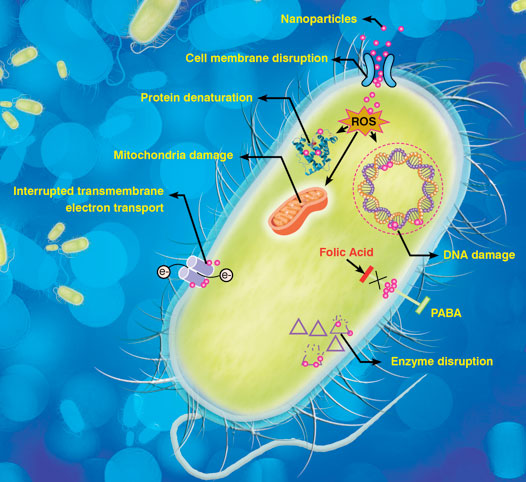 |
|
| Fig. 2: Probable mechanisms of nanomaterial based antibacterial solutions. | |
| Nanotechnology-based approaches for diagnosing superbugs | |
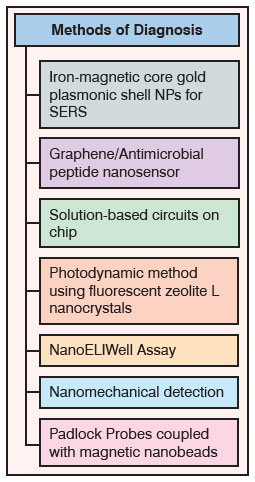
| Although several conventional techniques with high sensitivity and reproducibility are available to detect MDR infections, they are cumbersome and time consuming. Nanoscience can offer various accurate, economical and less time-consuming methods, which will help to avert microbial spread and its consequences. A team of researchers from Jackson State University, USA has developed a new popcorn-shaped iron-magnetic core gold plasmonic shell nanoparticles for surface-enhanced Raman spectroscopy (SERS) detection and photothermal destruction of MDR Salmonella bacteria. They have also reported that the same core-shell nanoparticle can be used in combination with near infrared (NIR) light to form light-directed nanoheaters for hyperthermic destruction of MDR12. | |
| In another approach, the solution-based circuit chip (SCC) for simultaneous identification of resistant and non-resistant E. coli causing urinary tract infections has been developed. These SCCs were fabricated by using a series of lithographic steps followed by electro-chemical deposition which creates 3-D nanoscopic morphology on microsensors. Further, these patterned microsensors were functionalized with peptide nucleic acid (PNA) probes to target specific region of pathogens. This multiplexed technique is also capable of distinguishing different bacterial strains like S. aureus and E. coli present in the same sample13. | |
| Similarly, Nguyen and the team from The Methodist Hospital Research Institute, USA has invented a novel NanoELIwell device for MDR-TB diagnosis. The technique is a combination of mycobacteria antigen immunoassay and microwell technologies. This device is able to perform fast identification and detection of antigens released by drug-susceptible and resistant mycobacteria14. | |
 |
|
| Table 1: Nanotechnology-enabled approaches for diagnosis of superbugs | |
| A different diagnostic approach was developed by using padlock probes (a type of linear oligonucleotide) coupled with magnetic nanobeads to detect rifampicin antibiotic resistant TB bacteria. These padlock probes were used to target the mutated genes present in rifampicin resistant bacteria15. | |
| A research team from Princeton University, USA has developed a graphene based nanosensor to detect pathogenic bacteria at the surface of biomaterials like tooth enamel. The specificity for biorecognition was achieved by attaching odoranin-HP (OHP–an antimicrobial peptide) peptide on graphene monolayers, which has a broad-spectrum activity towards pathogenic bacteria like MRSA16. | |
| Similarly, amino-functionalized Zeolite-L-nanocrystals conjugated with a green fluorescent dye and a photosensitizer has also shown potential for the detection of antibiotic resistant bacteria. These crystals when adhere to the surface of bacteria impart green fluorescence and make them visible. The nanocrystals also have abactericidal action, which is based on the ‘photodynamic principle’ (when exposed to light starts the process of killing bacteria)17. In another research, Longo and colleagues from EPFL, University Hospital of Lausanne and the University of Lausanne have proposed the nanomechanical detection by using low-frequency fluctuations of cantilevers when coated with antibiotic-resistant bacteria. This nanomechanical approach, which is correlated to the cell metabolism of microorganism in presence of antibiotic, is useful in identifying resistant bacteria within minutes18. | |
| Nanotechnology-based preventive measures against superbugs | |
| Recent advances in nanotechnology hold immense potential to modify surface properties, which can be exploited for targeting biofilms to control bacterial spread. EcoActive Surfaces, Inc. has developed a new mineral based photocatalyst nanocoating of Zn/titanium dioxide named as OxiTitan. This sol type coating can be easily applied to textiles and surfaces, which is cost- effective, durable and eco-friendly with proven action against Clostridium difficile spores and other antibiotic resistant bacteria. According to the evaluation studies it can reduce the infection of C. difficile up to ten times in 24 hrs19,20. | |
| Similarly, a research team from Rensselaer Polytechnic Institute, USA has developed a nanoscale coating for surgical equipments to target MRSA. This nanocoating is made up of carbon nanotubes combined with enzyme Lysostaphin (a natural enzyme, which attacks the bacterial cell wall causing its slicing). It is eco-friendly, as it does not leach out into environment with time. It is highly toxic to MRSA and has a dry shelf-life of around six months21. | |
| In another approach, scientists have developed nanoporous magnetic-like antimicrobial coating to trap and kill superbugs like S. aureus and P. aeruginosa. This coating is made up of positively charged dimethyldecylammonium chitosan methacrylate. The interaction with the negative charge present on bacterial cell wall result in the disintegration of cell wall, which causes cell death. This nanocaoting can be widely applied on biomedical objects like catheters, implants and contact lenses22. | |
| Another commonly prevalent problem where higher chances of infections are observed is associated with the development of biofilms. Bacteria, especially MRSA sticks to the surface of implants and form biofilm matrices, where they multiply and spread the infections. Nanotechnology can be used as an alternative approach against the accumulation of biofilms on the prosthesis and implanted devices23. Several nanomaterials such as AgNPs24, ZnO25, AuNPs coated with vancomycin26 have already shown good potential against antibiotic resistant E. coli, S. auerus and Vancomycin-resistant Staphylococcus aureus (VRSA). Magnesium fluoride NPs were found to be effective as a coating for glass to prevent the formation of biofilms on its surface27. | |
| Researchers have also reported a novel, nanomechanical approach through sensory applications of piezoelectric nanomaterials to investigate the workings of vancomycin – one of the last powerful antibiotics used to combat increasingly-resistant infections such as methicillin-resistant MRSA | |
| Nanotechnology-based therapy to combat superbugs | |
| General treatment of antibiotic-resistant bacteria requires multiple drugs regimen,which can cause many side-effects. Also, the treatment is costly and time consuming. Nanoscience can pave way for new treatment methods at much accelerated rate as compared to developing new antibiotics, which probably takes 10-12 yrs at an estimated cost of approximately $4 billion. | |
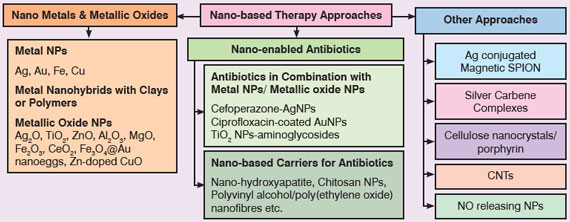 |
|
| Fig. 3: Nanotechnology-based therapeutic approaches to fight superbugs | |
| Metal and Metallic Oxide NPs: Metal NPs are potential candidates to deal with emergent MDR strains. Among metal NPs; silver NPs have shown maximum potential in different studies and proven to be effective against a broad spectrum of antibiotic resistant bacteria like MRSA, MRSE, erythromycin-resistant Str. pyogenes, ampicillin-resistant E. coli and multidrug-resistant P. aeruginosa28. | |
| Su and his co-workers from National Chung Hsing University, Taiwan have developed nanohybrids of AgNPs by adhering them with 1nm-thick inorganic silicate clay platelets. This improves the surface properties of AgNPs, reverses agglomeration and showed potent action on silver resistant E. coli and MRSA29. | |
| Studies also supported the use of poly-methyl-methacrylate (PMMA) to load silver nanoparticles for reducing its toxicity on mammalian cells30. Combination of AgNPs and polymer chitosan acetate has shown potential against MRSA when used in burn dressings31. Scientists have also used gold nanoclusters as potential antimicrobial against antibiotic resistant bacteria. They have prepared functional gold nanoclusters (Au NCs) by using lysozyme as sequestering and the reducing agent. These lysozyme–Au NCs were effectively inhibiting the growth of notorious antibiotic-resistant bacteria, like pan-drug-resistant Acinetobacter baumannii and vancomycin-resistant Enterococcus faecalis32. | |
| Lara et al., from Winston-Salem State University, USA has reported the use of silver oxide nanoparticles (Ag2O-NPs) as good antibacterial agent against multidrug-resistant bacteria like erythromycin-resistant Str. pyogenes, ampicillin-resistant E. coli, MRSA and MRSE33,34. In research studies performed at JNTU University, India, TiO2-NPs were found to be effective against antibiotic resistant E. coli35. TiO2-NPs were also able to enhance the activity of various antibiotics like beta-lactams, cephalosporins, aminoglycosides, macrolids etc. against MRSA36. ZnO-NPs were also found to possess good antibacterial action against MDR via targeting the bacterial cell membrane resulting in increased permeability leading to cell death. Further, polyvinyl alcohol coated ZnO-NPs also induce oxidative stress along with cell membrane targeting37. Multifunctional vancomycin immobilized Fe3O4@Au nanoeggs as photothermal agents were also effective in killing antibiotic resistant bacteria38. | |
| Nano-enabled Antibiotics: Researchers have also explored various nanosized carriers as drug delivery systems (DDS) for antibiotics which have shown proven effectiveness against antibiotic-resistant bacteria. A research group from Fudan University, China has prepared nano-hydroxyapatite (nHA) pellets as carrier for vanomycin and investigated its effectiveness against MRSA induced chronic osteomyelitis and other bone defects. It was found that nHA pellets act as a good bone graft material to reconstruct bone defects and to control bacterial infection39. | |
| Another study reported the synergistic effect of chitosan NPs with sulfamethoxazole against resistant P. aeruginosa40. El-Newehy and group from King Saud University, Saudi Arabia has used a novel technique of encapsulating antibiotic inside nanofibers made up of polyvinyl alcohol and polyethylene oxide, which actually boost up the power of antibiotics. The encapsulation controls the drug release thereby makes them effective for longer duration of time41. Combining antibiotics with metallic nanoparticles can also be a promising approach to overcome resistance in bacteria. Fayaz and co-workers from University of Madras, India have reported that antibacterial effect of cefoperazone against MRSA was increased when combined with AgNPs42. Similar effects were reported for vancomycin-capped AuNPs against VRE43 and ciprofloxacin-coated AuNPs44. | |
| Other Approaches: Schoenfisch and team at University of North Carolina, USA have demonstrated that silica nanoparticles releasing nitric oxide (NO) are effective against antibiotic resistant P. aeruginosa45. Similar results were obtained in another study where NO releasing nanoparticles had shown broad spectrum antibacterial effect on antibiotic resistant K. pneumonia, E. faecalis, Str. pyogenes, E. coli and P. aeruginosa. These NPs are actually targeting the bacterial cell membrane and changes its structure. Besides this, they also release reactive nitrogen species (RNS) which modifies the essential protein in bacteria causing cell death46. | |
| Scientists have also combined the unique properties of silver with superparamagnetic iron oxide nanoparticle (SPION) and targeted it against MRSA-biofilms. They reported for the first time that silver-conjugated SPION can be used as effective antibacterial agent to target the site of infection as well as to eradicate MRSA biofilms in the presence of magnetic fields47. | |
| Several other nanomaterial combinations were also explored by various research groups to fight against superbugs. Antimicrobial photodynamic therapy has been investigated as an alternative approach for treating microbial infections using cellulose nanocrystals modified with porphyrin-derived photosensitizer. These crystals were capable of inducing photodynamic inactivation of multidrug resistant Acinetobacter baumannii (MDRAB) and MRSA48. Silver carbene complexes (SCCs) encapsulated in poly(ethylene glycol)-poly(lactic acid) (PEG-PLA) nanoparticle complexes act as controlled release systems and were found to be active against various antibiotic resistant forms of bacteria like MRSA, P. aeruginosa, B. cepacia, K. pneumonia etc49. Some research also suggested the use of carbon nanotube (CNT) nanoclusters for antimicrobial photothermal therapy by delivering CNTs nanoclusters to the infected site followed by bacterial adsorption and selective destruction of antibiotic resistant bacteria by near IR irradiation50. | |
Conclusions |
|
| Antibiotics solely can never be a solution for superbugs and thus, scientists are exploring novel therapeutic approaches. Application of nanotechnology improves the effectiveness of antibiotics due to the following reasons: | |
|
|
|
| References | |
| 1. K.K. Kumarasamy, M.A. Toleman, T.R. Walsh, et al., The Lancet Inf. Diseases, 10(9) (2010) 597-602 | |
| 2. A.E. Clatworthy, E. Pierson, and D.T. Hung, Nat. Chem. Bio., 3 (2007) 541–548 | |
| 3. L. Einck, M.C. Mulvey, K. Sacksteder, “Methods and compositions for determining the pathogenic status of infectious agents”, US Patent No. 7919234 B2, Issue Date: April 5 (2011), Assignee: Sequella, Inc., USA | |
| 4. J.F. Blazyk, “Cationic, amphipathic beta-sheet peptides and uses thereof”, US Patent Pub. No. 2004/0249122 A1, (Filed on Feb 15, 2001) | |
| 5. S-J. Chen, M-C. Hsu, C-H. R. King, L. Lin, US Patent No.: US8211909 B2, Issue Date: July 20, 2012, Assignee: TaiGen Biotechnology Co., Ltd, Taiwan | |
| 6. M.S. Filutowicz, “Anti-microbial agents”, US Patent No. 7758854 B2, Issue Date: July 20, 2010, Assignee: Wisconsin Alumni Research Foundation, USA | |
| 7. WHO Report: Towards universal access to diagnosis and treatment of MDR-TB and XDR-TB by 2015, 23 March (2011) | |
| 8. BMJ Group blogs "Capreomycin shortage – symptom of a bigger problem in multidrug-resistant tuberculosis" | |
| 9. R.M. Klevens, M.A. Morrison, J. Nadle, et al., JAMA, 298(15) (2007) 1763-1771 | |
| 10. http://en.wikipedia.org/wiki/Antibacterial | |
| 11. P.M. Hawkey, BMJ, 317(7159) (1998) 657–660 | |
| 12. Z. Fan, D. Senapati, S.A. Khan, et al., Chem. Eur. J., 19 (2013) 2839-2847 | |
| 13. B. Lam, J. Das and R.D. Holmes, Nat Commun., 4:2001, doi:10.1038/ncomms3001 | |
| 14. Y.H. Nguyen, X. Ma and L. Qin, Scientific Reports, 2(635) doi: 10.1038/srep00635 | |
| 15. A. Engstrom, T.Z.G.de la Torre, M. Stromme, et al., Plos One, 8(4) (2013) doi:10.1371/journal.pone.0062015 | |
| 16. M.S. Mannoor, H. Tao, M.C. McAlpine, et al., Nat. Commun. 3 (763) doi: 10.1038/ncomms1767 | |
| 17. C.A. Strassert, M. Otter, R.Q. Albuquerque, et al., Angew. Chem. Int. Ed., 48 (42) (2009) 7928-7931 | |
| 18. G. Longo, L. Alonso-Sarduy, L. Marques Rio, et al., Nature Nanotechnology, 8 (2013) 522–526 | |
| 19. OxiTitan press release: OxiTitan Antimicrobial Coating Kills Deadly Clostridium difficile Spores on Surfaces | |
| 20. http://www.oxititan.com | |
| 21. R.C. Pangule, S.J. Brooks, C.Z. Dinu, et al., ACS Nano, 4(7) (2010) 3993–4000 | |
| 22. http://www.gizmag.com/antibacterial-coating-superbug-ntu/22505/ | |
| 23. E. Taylor and T.J. Webster, Int. J. Nanomed. 6(2011) 1463–1473 | |
| 24. J.R. Ellis, Am. J. Infect. Control, 35(5) (2007) E2 | |
| 25. K.R. Raghupathi, R.T. Koodali, A.C. Manna, Langmuir, 27(7) (2011) 4020–4028 | |
| 26. F.A. Mohammed, M. Girilal, S.A. Mahdy, et al., Proc. Biochem., 46(3) (2011) 636–641 | |
| 27. J. Lellouche, E. Kahana, S. Elias, et al., Biomaterials, 30 (2009) 5969–5978 | |
| 28. M.K. Rai, S.D. Deshmukh, A.P. Ingle, et al., J Appl. Micro., 112 (2012) 841-852 | |
| 29. H.L. Su, S.H. Lin, J.C. Wei, et al., PLoS ONE, 6(6) (2011) 1-10 | |
| 30. V. Alt, T. Bechert, P. Steinrucke, et al., Biomaterials, 25(18) (2004) 4383–4391 | |
| 31. L. Huang, T. Dai, Y.Xuan, et al., Antimicro. Agents Chemother., 55(7) (2011) 3432–3438 | |
| 32. W.Y. Chen, J.Y. Lin, W.J. Chen, et al., Nanomed., 5 (5) (2010) 755-764 | |
| 33. H.H. Lara, N.V. Ayala-Nunez, L. Ixtepan-Turrent, et al., World J. Microbiol. Biotechnol., 26 (2010) 615-621 | |
| 34. A. Nanda and M. Saravanan, Nanomedicine, 5 (2009) 452-456 | |
| 35. M. Haghi, M. Hekmatafshar, M.B. Janipou, et al., Int. J. Adv. Biotech. and Res., 3(3) (2012) 621-624 | |
| 36. A.S. Roy, A. Praveen, A.R. Koppalkar, et al., J. Biomat Nanobiotech, 1 (2010) 37-41 | |
| 37. Z. Huang, X. Zheng, D. Yan, et al., Langmuir, 24 (2008) 4140-4144 | |
| 38. W.C. Huang, P.J. Tsai, Y.C. Chen, Small 5 (1) (2009) 51-56 | |
| 39. L.L. Jiang, Y.F. Li, T.L. Fang, et al., Inflamm. Res. 61 (2012) 207-215 | |
| 40. S. Tin, K.R. Sakharkar, C.S. Lim, et al., Int. J. Bio. Sci., 5(2) (2009) 153-160 | |
| 41. ACS press release: Antibiotics wrapped in nanofibers turn resistant disease-producing bacteria into ghosts | |
| 42. A.M. Fayaz, K. Balaji, M. Girilal, et al., Nanomedicine 6 (2010) 103-109 | |
| 43. H. Gu, P.L. Ho, E. Tong, et al., Nano Lett., 3 (2003) 1261-1263 | |
| 44. R.T. Tom, V. Suryanarayanan, P.G. Reddy, et al., Langmuir, 20(5) (2004) 1909-1914 | |
| 45. E.M. Hetrick, J.H. Shin, N.A. Stasko, et al., ACS Nano, 2(2) (2008) 235-246 | |
| 46. A.J. Friedman, K. Blecher, D. Schairer, et al., Virulence, 2 (2011) 217-221 | |
| 47. N.G. Durmus and T.J. Webster, Adv. Healthcare Mater., 2 (2013) 165-171 | |
| 48. B.L. Carpenter, E. Feese, R.A. Ghiladi, et al., Photochem. Photobiol., 88 (2012) 527-536 | |
| 49. J.G. Leid, A.J. Ditto, A. Knapp, et al., J Antimicrb. Chemother., (2011), doi: 10.1093/jac/dkr408 | |
| 50. T. Maisch, Mini Rev. Med. Chem. 9 (8) (2009) 974-983 | |
|
By Deepika Bhatt, M. Kavitha, C. K. Nisha and Yashwant Mahajan, Centre for Knowledge Management of Nanoscience and Technology (CKMNT). For more information, interested readers may please contact Yashwant Mahajan at [email protected] or [email protected] and obtain a copy of the full-text article in pdf format.
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
