| Posted: Nov 13, 2017 | |
Atomic tuning of electrocatalysts |
|
| (Nanowerk Spotlight) Efficient electrocatalysts lie at the heart of a series of significant energy conversion and storage technologies, and atomically precise understanding of the influences of component dopants is crucial for looking into the reaction mechanism and controlled synthesis of the desired electrocatalysts. | |
| Graphitic carbon nitride (g-C3N4) – a stacked two-dimensional (2D) π-conjugated polymer consisting of repeating in-plane heptazine moieties – is a promising electrocatalytic material owing to its intrinsically high N content and abundant edge sites. This material has been researched towards some of the most significant electrocatalytic reactions including oxygen reduction/evolution reaction (ORR/OER) and hydro evolution reaction (HER). | |
| Over the past few years, scientists have discovered that diverse conductive substrates (carbon black, carbon nanotube/fiber, graphene and porous carbon) can be utilized validly to tackle the low conductivity of semiconductor-behaved g-C3N4, while nanodot, nanoribbon and nanosheet structures have been fabricated to unravel the influence of nanoarchitecture of this polymer on its different electroactivities. | |
| Although inspiring advances have been achieved, these arduous prior endeavors focused almost exclusively on the exterior modification over the g-C3N4 species (e.g., coupling g-C3N4 with various conductive substrates, and/or tailoring g-C3N4 into different architectures), which seldom involve the intrinsic properties of this material. | |
| The underlying role of another significant aspect – component dopants – towards the electrocatalytic performance is still quite elusive to date, which largely hampers the rational design and full exploit of this material in the electrocatalysis domain. | |
| A collaborative work by Prof. Chunyi Zhi from City University of Hong Kong and Prof.Zhongfang Chen from the University of Puerto Rico has comprehensively explored the influences of component elements within g-C3N4 motiety for electrocatalytic reactions. | |
| Their work – the first comprehensive investigation focusing on the influential component factor for g-C3N4 as metal-free materials in electrocatalysis domain – has been published in ACS Nano ("Component Matters: Paving the Roadmap Towards Enhanced Electrocatalytic Performance of Graphitic C3N4-based Catalysts via Atomic Tuning"). | |
| "Since g-C3N4 consists of repeating C and N sites, the first step is to substitute both sites alternatively by heteroatoms," explains Zengxia Pei, a PhD candidate from City University of Hong Kong, who served as the study's co-first author. "To address this issue, we synthesized three representative elements, B, P and S, embedded g-C3N4 samples, in which B/P substitute C while S replaces N site to coordinate with C. The involving of both sites in these samples then fundamentally ensures the evaluation of the general componential influences over their electrocatalytic activities." | |
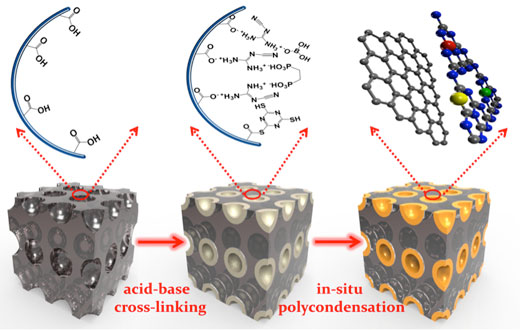
| The research team incorporated the doped g-C3N4 samples within a macroporous carbon substrate by an in situ polycondensation process, as illustrated in the schematic diagram shown below. | |
 |
|
| Schematic illustration for the fabrication of the PC supported B/P/S-doped g-C3N4 samples. For clarity, the separate B/P/S doping processes are incorporated into one framework. | |
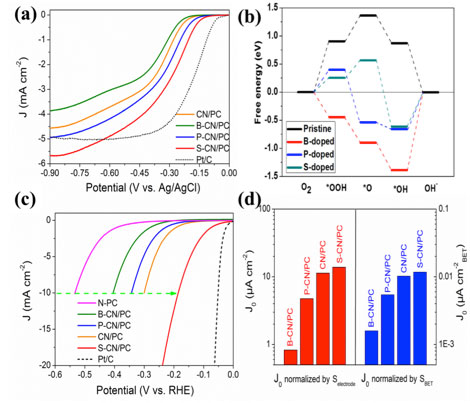
| "We found the dopant atoms have distinct effects on the ORR activity of g-C3N4 in alkaline electrolyte," Pei tells Nanowerk. "In experimental measurements, the S and P elements were found to boost the ORR activity of g-C3N4, while B-doped hybrid catalyst showed inferior catalytic performance compared with the pristine sample." | |
| To understand such experimental observations, the computational team performed DFT computations. Says Jinxing Gu, a PhD candidate from University of Puerto Rico, who served as the study?s co-first author: "our computations suggested that all the dopants could alter the reaction pathway of the g-C3N4 species for ORR. The rate-determining step on the most favorable reaction sites changed from the first electron transfer process to the last uphill reaction after doping by heteroatoms. The calculated activity follows the sequence of S-doped > P-doped > pristine > B-doped g-C3N4, which is in agreement with the experimental results." | |
| Nevertheless, the ORR involves merely the C sites. "To unravel the general influence of componential elements, we further assessed the HER activity of the samples in acidic solution because the residual N species serve as the adosorption sites for protons," Pei adds. | |
 |
|
| Influences of heteroatoms on the electrocatalytic activities of g-C3N4 material. (a) Polarization curves of different samples for ORR in 0.1 M KOH. (b) Free energy diagram of the most favorable sites within pristine and different atom doped g-C3N4 samples at the equilibrium potential U0 = 0.46 V. (c) HER polarization curves of different samples. (d) Normalized exchange current density (J0) of different samples indicating their intrinsic HER activities. | |
| The researchers found that the heteroatom embedded g-C3N4 samples also exhibited distinct HER activities in acidic solution. | |
| However, in the HER case, B and P elements were found to deterioate the activity compared with the pristine g-C3N4. | |
| The S species, most promisingly, could enhance the HER performance to deliver a metric reduction current density of 10 mA cm-2 at a small overpotential of -186 mV, which is comparable with that by the best non-noble metal HER-active MoS2 materials. | |
| "Our DFT calculations suggested that the optimized active site in the S-doped g-C3N4 for HER displays a small Gibbs free energy (ΔGH*) of only -0.03 eV, which is even better than that from the benchmark Pt/C catalyzer, suggesting the proper doping protocol is valid in boosting the HER activity of g-C3N4-based electrocatalyst," Gu tells Nanowerk. | |
| "Our collaborative work exemplifies that theoretical calculations is powerful for providing information on the electrochemical processes at the atomic/molecular level," summarizes Prof. Zhongfang Chen. "Joint electrochemical measurements and theoretical calculations can indeed be an effective strategy for elucidating the catalytic behaviors of a given material, which allows more precious unravelling of the fundamental mechanism of catalytic reactions and rational design of component-structure optimized electrocatalysts." | |
| "This work highlights the underlying role of component aspect within g-C3N4 material for electrocatalytic applications," concludes Prof. Chunyi Zhi. "We demonstrated that only proper doping is an effective strategy to greatly boost the electrocatalytic performance of g-C3N4. That is, in short, component matters, yet not every one works. We expect the results of this work to shed new light on the empirical trial-and-error exploration in design and development of g-C3N4-based materials as well as other metal-free catalysts for energy-related electrocatalytic reactions." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
