| Posted: Mar 01, 2018 | |
Controlled release of nanohydrogel from halloysite nanotubes |
|
| (Nanowerk Spotlight) Since the early days of nanotechnology in medicine, nanocarriers – nanostructures that are used to transport therapeutic compounds inside living organisms – have been attracting the interest of researchers because of their great potential in targeted drug delivery due to a wide range of possibilities for surface modifications and compatibilization. | |
| Another attractive issue of these nanoscale cargo systems is the ability to release the active molecules on demand (read more in some of the many Nanowerk Spotlights we have written on these issue: "Photo-triggered on demand drug release of nanoparticles" or "Electrically triggered drug release from smart nanomembranes" or ˆ"Remote-controlled nanocomposite for on-demand drug delivery inside the body". Or a very neat DNA origami nanorobot with a switchable flap to release cargo). | |
| A popular structure for the development of nanodelivery systems are hollow tubular nanoparticles. In new work, reported in ACS Applied Materials & Interfaces ("Nanohydrogel formation within halloysite lumen for triggered and sustained release"), researchers from Kazan Federal University and the University of Palermo show that a hydrogel can be confined within the cavity of halloysite nanotubes (HNTs) by means of an easy strategy. | |
| "The alginate network inside the HNTs cavity can be triggered by chemical stimuli (by calcium chelators) altering the kinetics, which results in the release of the cargo," Giuseppe Lazzara, professor, Department of Physics and Chemistry at the University of Palermo, tells Nanowerk. "With this work we have demonstrated that halloysite with tunable hydrophilic/hydrophobic interfaces can act as nanotemplate for the synthesis of drug delivery systems based on biopolymer hydrogels." | |
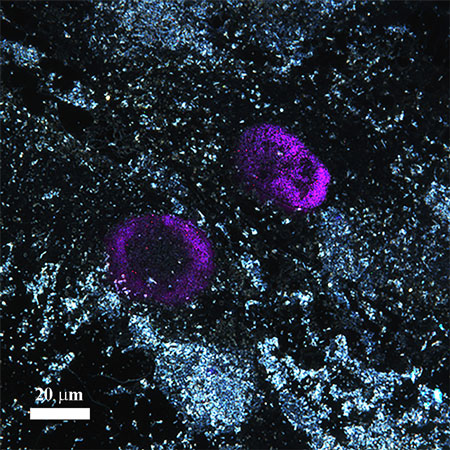 |
|
| Dark field and fluorescence microscopy image of distribution of modified halloysite nanotubes filled with the nanohydrogel in human cells. | |
| Current strategies regarding the use of hollow tubular nanostructures for drug delivery involve smart end-stoppers at the edges of these tubes that are broken under particular conditions (such as pH value) or triggered by external stimuli (such as light) in order to release the cargo. | |
| The fabrication of theses nanocarriers usually requires two steps – first the cargo is loaded and then the caps are attached to the tube openings in order to seal them. | |
| The novelty of the fabrication strategy of Fakhrullin's team is that it requires only one step. It is based on reverse inorganic micelles formed by halloysite nanotubes and their dispersions in oil phase. This approach enforces the confinement of alginate hydrogel within the halloysite, thereby generating a nanomaterial with promising properties for sustained delivery of active molecules in general. | |
| The team selected doxycycline, a broad-spectrum antibiotic, as model drug for loading and release from the confined hydrogel. The possibility of triggering the drug release from the hydrogel was exploited by adding ethylenediaminetetraacetic acid – which is a Ca2+ chelant – that can control the hydrogel rupturing. | |
| The confinement of the hydrogel within the halloysite cavity was imaged with advanced state-of-art methodologies (TEM micrographs and EDX elemental mapping, fluorescence experiments on marked polymers). | |
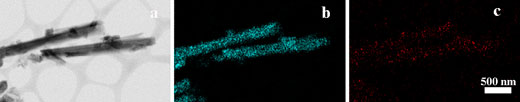 |
|
| Transmission electron microscopy image (a) and the corresponding EDX elemental mapping for silicon (b) and calcium (c) of modified halloysite nanotubes filled with the nanohydrogel. (click on image to enlarge) | |
| Biological tests on primary rat skin fibroblasts and human prostate cancer cell (PC3) demonstrated the biocompatibility of the hybrid hydrogel and the carrier distribution within the cells. | |
| "There are two main implications of our work," Rawil F. Fakhrullin, professor, Bionanotechnology Group, Institute of Fundamental Medicine and Biology, Kazan Federal University, notes: "First, a new easy strategy to control nanoarchitectures in hybrid inorganic/organic gel materials; and second, an alternative to end-stopper formation on nanotubular carriers for a further control of sustained/triggered release of the cargo." | |
| Next, the researchers plan a deeper investigation of the suitability of the hybrid nanocarrier for drug delivery by in vitro and in vivo experiments. Several drugs will be tested. | |
| Future investigations will also focus on the calcium and alginate release in cells and their effects in addition to the possibility of triggering the nanohydrogel disruption by chemical stimuli (pH or Ca ion complexing agents). | |
| The team's approach might open new avenues for the fabrication of a wide range of functional hybrid clay-organic materials. | |
| "Although our strategy is very promising in nanomedicine, we think that the features explored for such a system are promising for other applications as well, Fakhrullin concludes. "For instance, we are exploring the use of this methodology to develop smart protections for artwork that are relevant for the preservation of cultural heritage. Furthermore, the confinement of alginate hydrogel within the halloysite lumen might be a promising approach for the controlled cleaning of solid substrates. Another relevant application could be functional packaging obtained by the filling of polymeric matrices with the modified nanotubes. The nanocomposites might be suitable for long–term protection, as sensors and responsive materials to specific external stimuli." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
