| Oct 09, 2019 | |
The Periodic Table of Elements in the age of nanotechnology |
|
| (Nanowerk Spotlight) The Periodic Table of Chemical Elements is one of the most significant achievements in science, capturing the essence not only of chemistry, but also of physics, medicine, earth sciences and biology. 1869 is considered as the year of discovery of the Periodic System, and Dmitri Mendeleev was a major discoverer. | |
| Given that 2019 marks the 150th anniversary of the Periodic Table, the United Nations General Assembly and UNESCO have proclaimed the International Year of the Periodic Table of Chemical Elements (IYPT2019), celebrated with its own website. | |
| The periodic table is a unique tool, enabling scientist to predict the appearance and properties of matter on the Earth and in the the Universe. | |
| Elements are the fundamental substances from which all matters are composed. Currently there are 118 elements, with the first 94 elements all occurring naturally and the remaining 24 having only been synthesized in laboratories or nuclear reactors. | |
 |
|
| Simple periodic table chart. The image shows group/period in opposite order; periods are horizontal rows, groups are vertical columns. (click on image to enlarge) | |
| In the periodic table, periods are shown as horizontal rows and groups are shown in vertical columns. The elements in the same period have the same number of orbitals. Periods are characterized by the number of energy levels (shells) of the electrons surrounding the nucleus. The elements in the same group have the same number of electrons in the tilling orbit, and they have similar properties. | |
| "Different elements have played critical roles in different periods of human activities, with silicon (Si) being a key element, at present," Eugene A. Goodilin, a professor at M.V. Lomonosov Moscow State University, tells Nanowerk. "However, the nanotechnology age has brought different elements into the limelight and transformed their roles in science and technology." | |
| In a Perspective article in ACS Nano ("Nanotechnology Facets of the Periodic Table of Elements"), Goodilin together with professors Paul S. Weiss and Yury Gogotsi briefly discuss the most relevant elements from a nanotechnology perspective and their applications in nanomaterials. | |
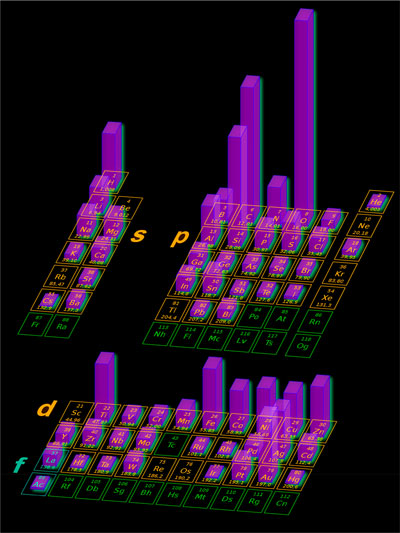
| The authors found that the most in demand and useful nanotechnological elements are concentrated in the s- and p-blocks (see figure below) with predominant nano elements being nonmetals C, N, O, and Si in the p-block and H and Li in the s-block. | |
 |
|
| Frequency of usage of chemical elements of the periodic table in nanotechnology. The plot is drawn using expert opinions and analysis of ACS Nano publications; higher neon glow bars correlate with the elements’ contribution to the nanotechnology field; green cells denote radioactivity; and the f-element families are not shown, they are marked with “La” and “Ac”. (Reprinted with permission by American Chemical Society) (click on image to enlarge) | |
| "Atomic number growth in table periods results in the compaction of the atomic radii and an increase of ionization potential," explains Goodilin. "Also, p-electrons come into play in the second period of the table of elements, leading to the formation of strong, oriented covalent bonds for most compounds of B, C, N, and O and anion formations for electronegative O and F. p-Electrons lead to conjugated π-bonds in addition to σ-bonding, and this new ability leads to a large number of inorganic polymers." | |
| The authors point out that "a major lesson from nanotechnology is that an element's individuality is more important than periodicity; the known 'similar' elements are actually nanotechnologically dissimilar and, in most cases, are not replaceable as we used to imagine in traditional sciences. Thus, most elements, except those in the common nanomaterials, have narrower but still important fields of use in nanotechnology." | |
| As a result, about 30 of the 118 primary elements are in high demand for various one-, two-, and three-dimensional (1D, 2D, 3D) nanomaterials, which are formed for B, C, and N, including graphene- or BN-mimicking analogues, nanotubes, fullerenes, MXenes, C3N4, nanodiamonds, etc. | |
| This group is the largest and fastest growing class of nanomaterials initiated through the nanotechnology revolution and the related meteoric rise of nanotechnological architectures used in the creation of supercapacitors, batteries, molecular electronics, fuel cells, sensors, and advanced construction materials. | |
| "The 150 year-old periodic table of the elements helps us appreciate the chemical diversity of elements in the search for effective elemental combinations to produce new nanomaterials, " Goodilin, Weiss and Gogotsi conclude. "The diversity of elements in the periodic table will enable further innovative developments in nanoscience and nanotechnology." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
