| Feb 21, 2023 | |
Turning food and plastic waste into valuable nanomaterials for energy applications |
|
| (Nanowerk Spotlight) Our society generates staggering amounts of waste in all areas of economic activities. Foremost among them, apart from energy waste, are the food and plastic sectors. | |
| Data gathered by the FAO (pdf), the Food and Agriculture Organization of the United Nations, estimates that around 931 million tonnes of food waste was generated in 2019, 61% of which came from households, 26% from food service and 13% from retail. This suggests that 17% of total global food production may be wasted. More than half of that is made up of fruit waste. | |
| According to a 2022 report by the World Economic Forum, the world produces about 400 million tons of plastic waste each year, but only 9% of that plastic is being recycled; 12% is incinerated and a whopping 79% is dumped in landfills or the environment. | |
| However, both food and plastic wastes are potentially valuable sources of carbon. In previous reporting we have covered various approaches by research teams around the world to turn food and plastic waste into feedstock for making nanomaterials or even make nanomaterials like graphene directly via flash synthesis. | |
| Edison H. Ang, an Assistant Professor at Nanyang Technological University Singapore, and his group are working on upcycling of waste materials to high-value carbon by combining materials science and nanotechnology approaches to develop functional nanostructures for advanced energy storage, catalysis, water purification, and biosensor applications. | |
| The group recently published two papers where they describe routes for the sustainable production of MXene from fruit waste (Chemistry - A European Journal, "Sustainable Production of Molybdenum Carbide (MXene) from Fruit Wastes for Improved Solar Evaporation") and the sustainable development of graphitic carbon nanosheets from plastic wastes (Journal of Materials Chemistry A, "Sustainable development of graphitic carbon nanosheets from plastic wastes with efficient photothermal energy conversion for enhanced solar evaporation"). | |
| "Both MXene and graphite are conductive in nature and their 2D structure makes them attractive to be used in energy storage applications," Ang tells Nanowerk. "Our primary goal with this research has been to create innovative and sustainable materials for constructing solar evaporators. Our aim here has been to use environmentally friendly methods to produce freshwater using solar energy. However, the challenge lies in identifying suitable renewable materials for this purpose. Hence, our focus has shifted to waste materials that can undergo carbonization and upcycling to create solar evaporators that are both environmentally friendly and more efficient." | |
| In their report in Chemistry - A European Journal, the team presents a straightforward, two-stage calcination process that enables the creation of two-dimensional (2D) layered molybdenum carbide (Mo2C) materials using fruit waste as the carbon source. The chosen fruit waste materials for this study were coconut husk, orange peel, and banana peel. These were selected because a significant proportion of the fruit (50-65% of the total mass) is inedible and is typically discarded as waste. | |
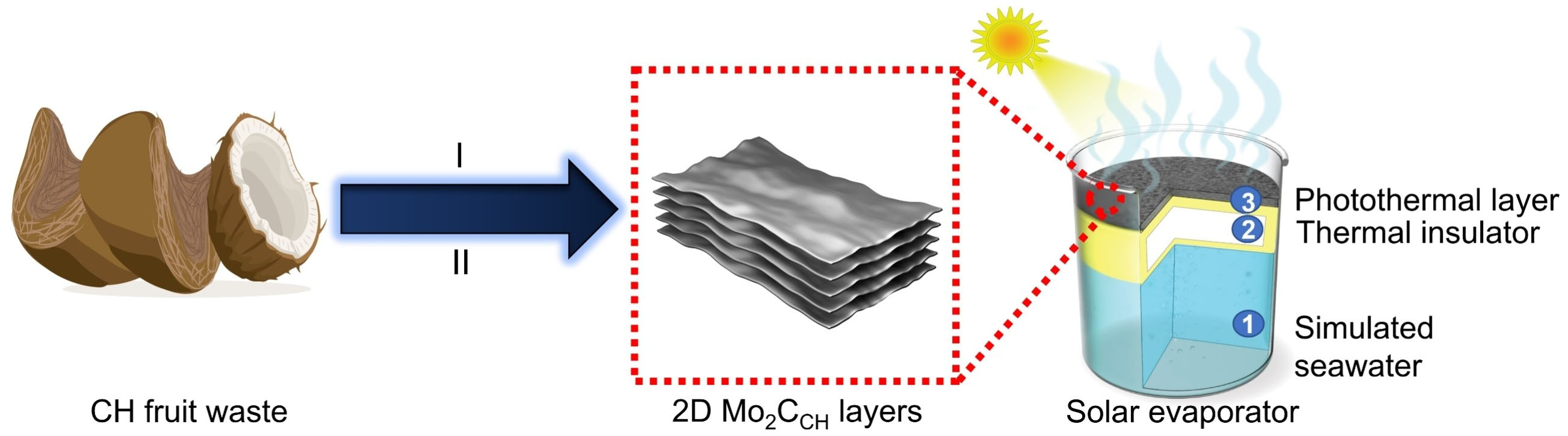 |
|
| Schematic illustration of the formation of 2D Mo2C layers from the recycling of coconut husk (CH) fruit waste. Stage 1: synthesis of carbonaceous materials derived from CH, denoted as CCH. Stage II: synthesis of 2D Mo2CCH layer by carbonization of CCH with Mo precursor. A common setup of Mo2CCH solar evaporator consists of three components, including the simulated seawater, the thermal insulator (i.e., polystyrene foam) along with the photothermal layer comprises of the 2D Mo2CCH layer deposited on the air-laid paper. (Reprinted with permission from Wiley-VCH GmbH) | |
| According to the researchers' preliminary findings, different types of fruit wastes have different water evaporation rates and photothermal conversion efficiency (PTCE) in solar water evaporators. The photothermal layer made from coconut husk has the highest PTCE of 94% and the highest evaporation rate of 1.52 kg m-2h-1 under one sun illumination (i.e., the amount of solar radiation that reaches the Earth's surface under normal conditions when the sun is directly overhead). | |
| "The large specific surface area of 555.1 m2g-1 and wide solar absorption band ranging between 300 to 1600 nm results in enhanced PTCE, while the better wetting ability and presence of a broad group micro- and mesopores enable rapid water transportation," Ang explains the results. "When compared to prior published data, this is the first time that such enhanced PTCE and evaporation rates are attained." | |
| In their report in Journal of Materials Chemistry A, the team demonstrates a simple two-step method involving acid treatment and carbonization to synthesize ultrathin (with a thickness of less than 1 nm) honeycomb-structured 2D graphitic carbon nanosheets (g-CNS) from plastic waste such as plastic bags and bottles. | |
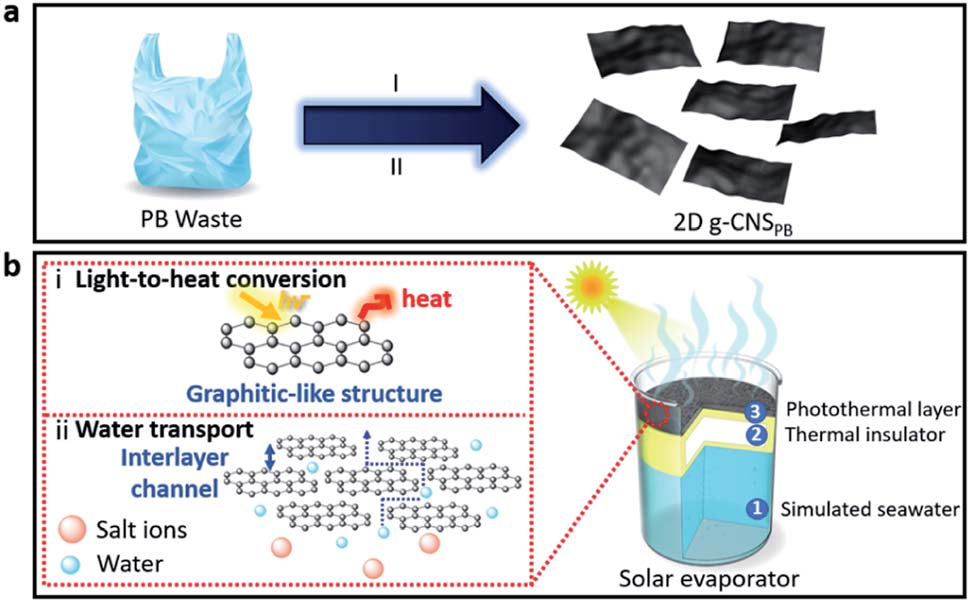 |
|
| (a) Schematic illustration of the formation of 2D graphitic carbon nanosheets (g-CNS) from upcycling of plastic bag (PB) waste. Stage I: growth of sulfonated carbon black derived from plastic bag (s-CBPB). Stage II: formation of 2D g-CNSPB by carbonization of s-CBPB. (b) The schematic shows a typical setup of a solar evaporator and the unique features of the 2D g-CNSPB consisting of: (1) simulated seawater, (2) a thermal insulator (i.e., polystyrene, PS foam), and (3) a photothermal layer made up of 2D g-CNS on an air-laid paper support. (Reprinted with permission from The Royal Society of Chemistry) | |
| "We believe this is the first time these graphitic 2D CNS were fabricated from plastic waste," says Ang. "The unique graphitic-like and 2D structures do not appear in previously reported carbonaceous materials originated from plastic waste. Because of the merits of the graphitic-like characteristics and the 2D interlayer channel architecture this can improve the light-to-heat conversion as well as the water transport for solar evaporation, respectively." | |
| In the next stage of their investigations, the team will work on extending the MXenes and graphite nanosheets recycled from organic wastes to other possible applications such as electrode materials for energy storage devices. | |
| "The challenges we face in extending this work to energy storage applications is to remove the impurities in the fruit and plastic waste since they may affect the performance of battery electrodes," Ang concludes. "We therefore need to develop methods to purify the feedstock materials in order to produce high-quality MXenes and graphite suitable for energy applications." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
